In Pakistan, the first case of COVID-19 was reported on 26th February 2020. Since then, there have been 1.5 million cases to date resulting in 30,307 deaths (case fatality rate of 2.0%).1,2 Vaccines against COVID-19 were made available in Pakistan in February 2021 and so far, around 75% of the eligible population has been fully vaccinated.3 Karachi has been the epicenter of the pandemic in Pakistan, and we have previously reported three serosurveys from the start of the pandemic. The first three cross-sectional surveys were performed sequentially in April, June – July, and August 2020 respectively.4 Here, we report results from three additional surveys, referred to as surveys four, five, and six, carried out in November 2020, February 2021, and December 2021, respectively, in one urban setting in Karachi, Pakistan.
METHODS
The study was done in the District East of Karachi.4 We used a systematic random sampling technique the details of which have been described previously. Briefly, an index household was randomly identified from the line listing of cases in the union council. This served as the reference point from where the households in the area were approached. To locate the first household, the team spun a bottle or pen to ascertain the direction. The ‘nth’ household to be approached was selected using a digit on a banknote. For each survey, different households were approached and recruited. All household members were eligible to participate regardless of age and infection status. Approval from the head of the household and written informed consent or assent, as appropriate, from individual participants was obtained to detect antibodies against SARS-CoV-2, Elecsys Anti-SARS-CoV-2 immunoassay targeting both IgG and IgM was performed.
For the fifth and sixth surveys, we collected additional information about the vaccination status of the participants, including number of doses with the corresponding dates. This information was ascertained through the government issued vaccination cards. If the vaccination cards were not available, then the information was based on participants’ recall. We classified an individual as fully vaccinated if 14 days had elapsed since the last dose of a single dose or two dose regimens.5
The target sample size in each survey was 500 individuals to allow for the estimation of age-adjusted seroprevalence in the range of 20–30% with 95% confidence, a margin of error of ±5% with a design effect of 1.5 for clustering at the household level. We used a hierarchical Bayesian regression model to estimate the age and gender-stratified seroprevalence. This model also adjusts for other factors such as the reported test accuracy and number of household members. The conditional risk of infection (CRI) was calculated as a fraction, with the total number of ordered pairs among infected individuals in the same household taken as the numerator, and the total number of ordered pairs in the same household, in which the first individual in the pair is infected, taken as the denominator. Confidence intervals were estimated via the bootstrap method.
RESULTS
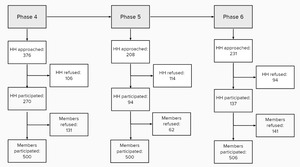
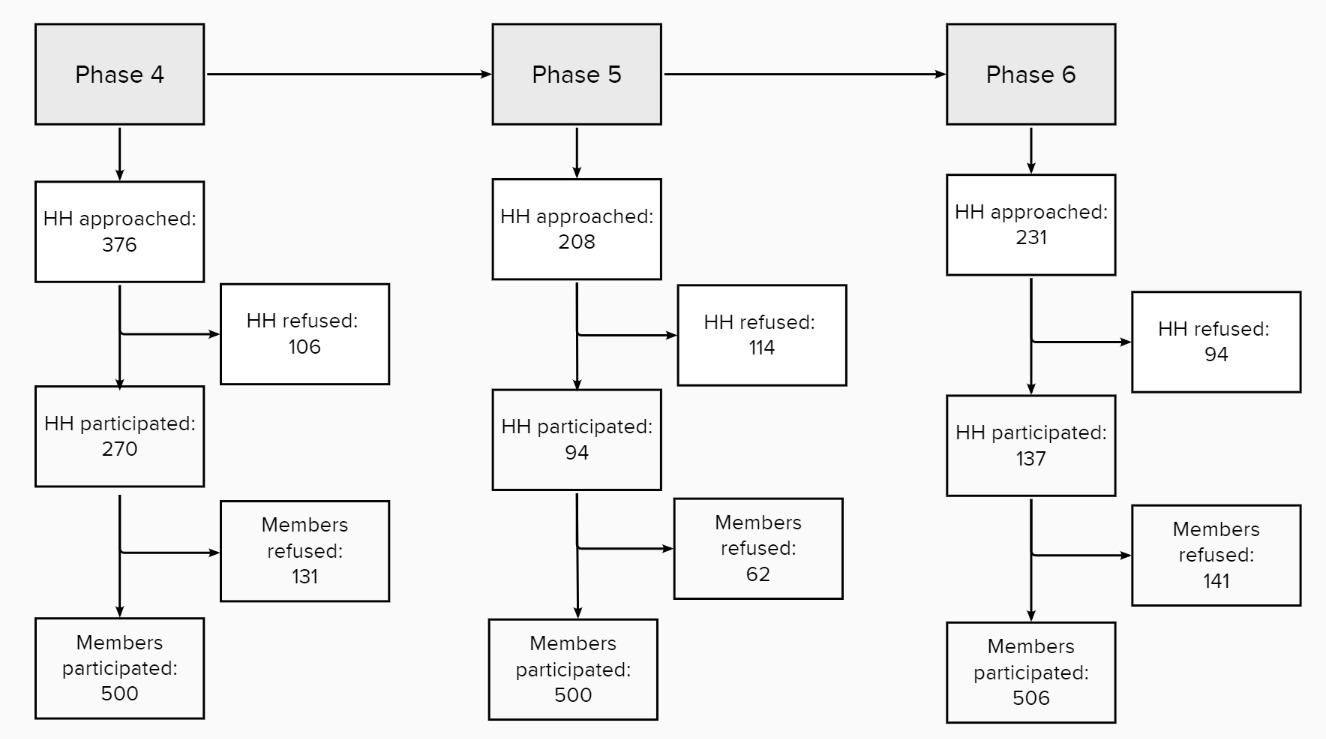
Of the households contacted, 28%, 55%, and 41% agreed to take part in surveys 4, 5, and 6, respectively. Within a household, individual participation rates were 79%, 89%, and 78%, respectively (Figure 1).
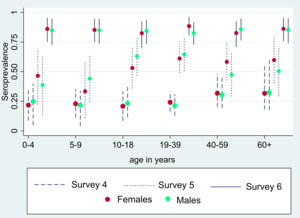
Post-stratified and adjusted seroprevalence increased from 24.0% (95% confidence interval, CI=18.0%-31.0%) in serosurvey 4 to 53.9% (95% CI=45.5%–63.2%) in serosurvey 5 and 84.9% (95% CI=78.5%-92.3%) in serosurvey 6. In comparison to the previous three serosurveys, the trend of seroprevalence, in district east, greatly increased over time (Figure 2). There were no significant differences in age and gender-specific seroprevalence rates in each survey (Figure 3 and Table S1 in the Online Supplementary Document). A high proportion of asymptomatic infection was reported in the 4th and 5th surveys (93.0% and 91.8%, respectively) which declined to 69.8% in the 6th survey (Table 1 and Table S2 in the Online Supplementary Document). Among the participants of the 6th survey, 31.2% (n=158) were fully immunized with a COVID-19 vaccine. The most common COVID-19 vaccines used were the two-dose inactivated vaccines, Sinopharm (37.6%) and Sinovac (36.2%) (Table S3 in the Online Supplementary Document) CRI was estimated to be 41% (95% CI=29.9-51.6), 56.7% (95% CI=50.4–62.6), and 77.8% (95% CI=73.0-81.7) in surveys 4, 5 and 6 respectively.
DISCUSSION
We describe an increasing seroprevalence of SARS CoV-2 across three serial surveys conducted between November 2020 and December 2021 in District East, Karachi, Pakistan. This trend is in continuity with the three surveys previously reported from the same district.4 We show that within two years of the first detected cases in Karachi, nearly 85% of the population had detectable antibodies against SARS-CoV-2. The outbreaks with earlier variants may have been milder, as evident through a higher proportion of asymptomatic infection. However, there was an increase in symptomatic cases in the last survey, a potential consequence of the preponderance of the delta variant prior to the 6th survey, and its association with more severe disease.6
There was no difference in seroprevalence across different age groups and genders. This is in line with previously published literature7 and strengthens the argument that everyone is equally vulnerable to the infection, including children.8
The high cumulative seropositivity should be interpreted with caution since it may not necessarily translate into future protection as emerging variants may develop mutations that allow for immune escape. Moreover, humoral immunity through natural infection may not be long-lasting. A sharp decline of IgG titers within 2-4 months has been reported,9 while IgA and IgM decline within 18 days after symptom onset.10 Even in the current era, vaccines do not augment the immune response to the extent that high levels of protection against infection are achieved. Thus, despite the high seroprevalence, a resurgence in the same community may still be observed.11 Our study had some limitations. For one, we used a qualitative test for antibody detection that does not distinguish between past and active infection, nor does it differentiate between antibodies developed through natural infection vs vaccination. Second, our study area was also restricted to a small geographical area within Karachi that may affect the generalizability of our results. However, Bergeri et al. in a systematic review, provided similar anti-SARS-CoV-2 antibody prevalence estimates globally during the same period, attributed in part to the rapid spread of the disease and partly to vaccination.12 The main strength of our study was that we captured the seroprevalence rates after each successive ‘wave’ of infection in Karachi, demonstrating that, despite the potential waning of titers, seroprevalence exhibited a clear increasing trend following each wave.
CONCLUSIONS
The study shows the value of community-based seroprevalence surveys in estimating the actual proportion of the population that have some level of immunity due to prior infection or vaccination. This information in turn may be helpful in predicting the pattern of spread of the disease in similar settings.
Acknowledgements
The authors would like to acknowledge all the data collectors, phlebotomists and laboratory personnel who made this happen in the most difficult of circumstances. The authors like to thank the leadership and staff at the District Health Office (District East) for their support.
Ethics approval and consent to participate
Study was performed in accordance with the ethical principles for medical research involving human subjects and was approved by Ethical Review Committee of The Aga Khan University, Karachi, Pakistan. Reference number is 2021-3685-20047. Written informed consent was taken for adult participants and for minor participants, written informed consent was taken from parents or legal guardians.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study was supported by the Infectious Disease Research Laboratory (IDRL) at Aga Khan University in Karachi, Pakistan.
Author contributions
Study Concept: FJ, IN. Study Design: FJ, IN, MA, NA, AH, AS, JI. Data Collection: FJ, IN, MA, NA, AH, AM, UM. Data Analysis: FJ, IN, MA, NA, FK, NR, DL, BF. Writing: FJ, IN, MA, NA, DL, BF
Competing interests
The authors completed the Unified Competing Interest form at http://www.icmje.org/disclosure-of-interest/ (available upon request from the corresponding author), and declare no conflicts of interest.
Additional material
This paper has an Online Supplementary Document.
Correspondence to:
Dr. Fyezah Jehan, Associate Professor, Department Chair, and an Infectious Diseases Specialist, Department of Paediatrics and Child Health, Aga Khan University, Stadium Road, Karachi 74800, Pakistan. Tel: +92 21 3486 4981; email: [email protected].