Cervical cancer (CC) is the fourth most diagnosed cancer and the fourth leading cause of cancer death among women globally.1 In the Philippines, CC is the second leading cause of cancer in women. In 2018 there were 9.1% new CC cases in the Philippines, compared to 0.47% new cases in Australia.1 Human papillomavirus (HPV) is a significant co-factor of CC. Almost all CC cases are caused by persistent infection with HPV.2 There is no national HPV immunisation schedule in the Philippines.3 Additionally, there is no systematised cervical cancer screening program through cervical cytology (Pap) or acetic acid visualisation (VIA/VILI)..3
About 75% of women diagnosed with CC in the Philippines are diagnosed with late-stage disease, attributing to the high mortality rate.4 Filipino women have a higher incidence of risk factors for CC than in the global community.5 Currently, there are three screening methods for the early detection of CC: (i) Pap test; (ii) VIA/VILI; and (iii) HPV test. Pap test is the oldest and most widespread CC screening technique. In developed countries the Pap test has led to a reduction in the incidence of CC. However, due to lack of resources, it has failed to have the same success in developing countries.6–8
Visual inspection tests with 3% - 5% acetic acid (VIA/VILI) is an alternative to cytology. This approach has been shown to reduce mortality in developing countries.9,10 HPV testing has been shown to be more effective than cytology for CC screening.11 Several studies demonstrate that HPV testing is the best strategy for reducing CC in low-resource settings.12–14
This audit is an extension to the previously published audit, completed in 2020 by Hespe and Carr.15 The previous audit analysed medical records collected during nine separate women’s health clinics in a regional community of Calauan, Laguna, Philippines. The audit was conducted prior to HPV-DNA testing being available. As a result, it investigated the prevalence of VIA/VILI abnormalities in the clinic and compared this to known risk factors of CC. Additionally, Hespe and Carr’s original audit investigated demographics of patients participating in the clinic, and the retention rates of the clinic.
No statistically significant relationships were identified, limited mainly by the small sample size and the low number of abnormal results. Without a gold standard comparison such as Human Papilloma Virus – Deoxyribonucleic Acid (HPV-DNA) testing, the sensitivity and specificity of the VIA/VILI method for cervical screening were unable to be evaluated.
This current audit includes results of HPV-DNA testing in 284 women, which had not been considered in the previous study and compares it with the VIA/VILI inspection results of the same 284 women (see Appendix A in the Online Supplementary Document). Our aim was to determine the prevalence of HPV in a rural community in the Philippines and compare HPV testing to the previously used visual inspection method.
METHODS
Study design
This study is a retrospective audit of data from medical records collected during 13 separate weeks of Women’s Health clinics conducted in July and November from 2013-2019 in a regional community of Calauan, Laguna, Philippines. The women who participated in the Women’s Health clinics consented to undergoing a CC screening procedure and using their de-identified data for future research and review.
The specific objectives were: (i) to determine the demographics of women in the target population (aged 16 - 72 years) who participated in the CC screening program between July 2013 and November 2019 in Calauan, Laguna, Philippines; (ii) to quantify the proportion of women screened between July 2013 and November 2019 identified as having “no cervical abnormality”, “low-grade changes/carcinoma insitu (LSIL/CIN 1)”, “high-grade changes (HSIL/CIN2 or 3)”, or “cervical cancer (CIS)” from July 2013 to November 2019 based on their VIA/VILI results; (iii) to determine the prevalence of HPV and particular HPV strains in the population that received HPV-DNA cytology from July 2018 to November 2019; and (iv) to compare results from the VIA method and the HPV-DNA cytology in patients screened from July 2018 to November 2019.
Recruitment
This research complies with the Australian National Health and Medical Research Council (NHMRC) National Statement on Ethical Conduct in Human Research. A Human Ethics Research Committee (HREC) low-risk ethics approval from the University of Notre Dame Australia was obtained as the project was a clinical audit of medical records and carried a low risk as per paragraphs 2.1.6 and 2.1.7 of the National Statement (HREC reference: 016204S). The approval date for the original audit was: 14/12/2016. Amendment date for additional HPV audit assessment was: 03/02/2020. No participant involvement was required in the audit review and data analysis and all data was non-identifiable. From July 2013 to November 2019, women aged between 16 to 72 were included in the audit. All women who participated gave informed consent. Based on their screening results, women were given follow-up advice. Patients’ adherence to follow-up was recorded.
Data collection
Eligible patients entering the clinic completed a paper record (See Appendix B in the Online Supplementary Document). Patients recorded their age, gender, height, weight, smoking status, number of sexual partners, contraceptive use and last menstrual period. Once the clinician inspected the patient’s cervix and applied the acetate, the experienced clinician recorded the VIA/VILI procedure result. Clinics one to nine were held from 2013 to 2017. This was prior to the availability of HPV-DNA testing in the clinic. As a result, these women only underwent VIA/VILI screening. Clinics 10 to 13 were held from 2018 to 2019. These women had both HPV-DNA testing and VIA/VILI screening. The HPV cytology was given a unique identifier, which correlated with the identification recorded on their paper record (see Appendix B in the Online Supplementary Document). The HPV cytology was securely transported to Australia by the research team and analysed in a Melbourne-based pathology laboratory.
Data analysis
Data collected from the Women’s Health clinics from July 2018 onwards were added to the de-identified dataset from the first audit conducted by Carr and Hespe15 onto an Excel spreadsheet. De-identified demographic and clinical data were exported from the Excel spreadsheets into STATA for analysis. Demographics were reported using frequencies and 95% confidence intervals. A two-by-two table was used to analyse the sensitivity and specificity of colposcopy.
RESULTS
A total of 872 women aged 16-72 presented to the Women’s Health clinic for CC screening over 13 weeks. None were excluded due to eligibility criteria. From 2018 HPV-DNA testing was available and 284 HPV tests were collected. Demographic characteristics and results of baseline screening are reported in Table 1.
Colposcopy results
From July 2013 to November 2019, 872 women underwent VIA/VILI screening in the Women’s clinic. Of those, 11% (98) were abnormal. This is consistent with the previous audit, which found 11% of VIA/VILI of 615 women to be abnormal.15
In our current audit, of the 872 women, 756 (86.7%) had normal colposcopies, 90 (10.32%) had CIN1 changes, 5 had CIN2/CIN3 changes (0.6%) and three had suspected cancer (see Table 2). From July 2013 to November 2019, three women were diagnosed with cervical cancer. They were all referred to the Manila General Hospital and received prompt investigation and treatment.
HPV results
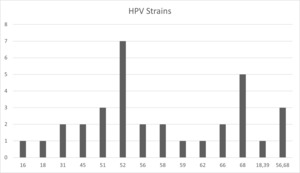
From July 2018 to November 2019, HPV testing was introduced into the Women’s clinic. HPV was detected in 32 (11%) of the 284 women tested. A wide range of different HPV strains were detected in these samples. Both types 16 and 18 were detected, along with types 31, 39, 45, 51, 52, 56, 62, 66 and 68 (see Figure 1).
Demographic results
The women in the community of Calauan report a higher use of contraception (45%) compared to the national Filipino average (40%).16 This is consistent with results found in the previous audit in this community.15 Filipino women have a significantly lower contraception rate than Australians, which in 2019 was reported as 70%.17 Filipino women face many barriers to contraception, including health concerns about contraception methods, fear of side effects, religious reasons and not being covered by the national health insurance program PhilHealth.18 As reported in the previous audit,15 women in Calauan continue to have a high fertility rate of 3.1 live births/women, compared to the national total fertility rate recorded in 2017 of 2.7 live births/woman.16 This is high compared to rural women overall, where the reported fertility rate is 2.8 live births/woman compared to an urban total fertility rate of 2.4 live births/woman.16
Overall, women in the Calauan clinic have more live births than rural women, despite their reported contraception use being higher. This disparity may be attributed to health literacy, with poor implementation of effective contraception use. Some women can only afford to take the oral contraceptive pill (OCP) intermittently, making it ineffective. In 2017 health education sessions run by the research team began focusing on fertility and contraception and over 300 women participated in these education sessions. Future studies could focus on interviewing the women in Tagalog (local language) to qualitatively determine their use of contraception, barriers to contraception and understanding of the effectiveness of contraception.
Compared to the previous audit, smoking rates rose from 11.8%15 to 12.4%. This is much higher than the reported national average of 4.9%. As per the previous audit, we could not provide data on the average number of cigarettes smoked. We have assumed, given anecdotal evidence, that most of these women cannot afford to smoke more than three cigarettes a day.
DISCUSSION
This study was an extension of a clinical audit of a CC screening program in the rural socio-economically deprived community of Calauan, Laguna, Philippines, conducted in 2018 and reported by Carr and Hespe.15
The main messages found in this study were: Thirteen different HPV subtypes exist in this community, all of which are oncogenic19; The economical bivalent HPV vaccination has a low coverage of the strains found in this community; The VIA/VILI screening method had a 100% specificity but low sensitivity (15%) in detecting abnormal cervixes; In rural communities, follow-up is hindered due to logistics. As a result, point-of-care (POC) testing and immediate referral are crucial for optimal care delivery.
HPV strains and vaccination options
Following publication of the previous clinical audit in this community,15 HPV-DNA cytology became available. All the HPV subtypes detected in this study were oncogenic.19 Importantly, none of the three available HPV vaccines covers all of these strains. There are currently three HPV vaccines in use worldwide: nonavalent HPV vaccine (Gardasil 9); quadrivalent HPV vaccine; and bivalent HPV vaccine. The bivalent vaccine only protects against HPV types 16 and 18, whereas Gardasil 9 protects against types 6, 11, 16, 18, 31, 33, 45, 52, and 58. These results are significant to note when considering a nationwide vaccination program. The three available vaccines have different price points: bivalent Cecolin vaccine costs $95.4USD; quadrivalent HPV Gardasil costs $360USD; and nonavalent HPV Gardasil 9 costs $586USD.20
This price difference is significant when considering vaccine choice for a population as large as the Philippines (110 million). This audit found HPV types 16 and 18 account for only 6% of the HPV-positive cases in the clinic. As a result, immunising using the cheaper bivalent vaccination ($95.4USD) would potentially be ineffective at preventing CC in this community. From the current data, the nonavalent vaccination ($586USD) could protect 81% of the HPV strains detected in this rural community.
Specificity and sensitivity of the VIA/VILI method
The VIA/VILI method was seen to be 100% specific; all patients found to have an abnormal VIA/VILI were also positive for HPV (see Table 4). The disadvantage of using VIA/VILI was the low sensitivity of 15%. This study suggests that HPV-PCR testing is superior at detecting HPV before cervical changes occur. This testing method detected 28 patients with a positive HPV test not otherwise seen using VIA/VILI method. As a result, these patients have an opportunity for earlier intervention and access to appropriate care. This audit shows the utility and benefit of using HPV-DNA cytology over the VIA/VILI method for early detection, referral and follow-up of HPV-positive cases.
Another strength identified from this audit is the clinician’s ability to detect abnormal cervixes using the VIA/VILI method, with no false positives detected. This method, therefore, continues to have advantages within communities unable to access Pap tests. No patients were inappropriately identified as being high risk and were not exposed to undue health anxiety. All “abnormal” cervixes detected using the VIA/VILI method were confirmed to have an HPV infection and needed to be provided with ongoing monitoring and appropriate care.
Ultimately, this clinical audit exemplifies the importance of using HPV-DNA cytology testing for the early identification and prioritisation of women at risk of CC.
Point of care testing
The Women’s Health clinic in Calauan, Laguna utilised free HPV testing via donated supplies and clinician oversight by a Melbourne-based pathology laboratory. As a result, patients tested in the Women’s clinic in July did not receive their HPV results or clinical follow-up until the next clinic held in November. Patients with abnormal VIA/VILI results were able to be referred to have a biopsy on the same day. However, as previously noted, VIA/VILI testing failed to detect another 28 high-risk cervixes. An alternative option is point-of-care (POC) tests, which provide rapid on-site results, and in resource-limited settings, this supports timely and proper treatment.21
Currently, two main POC tests are available for HPV detection: CareHPV22 and Xpert HPV.23 Both tests detect the high-risk HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.22,23 These tests retail for $23.61USD, $21.49USD and determine whether a patient has an oncogenic HPV strain in 2.5 hours for CareHPV and 1 hour for Xpert HPV.24 Patients can be diagnosed with HPV infection and referred for further testing on the same day of their appointment using POC tests. POC tests offers a solution to HPV testing that otherwise requires expensive laboratories, a reliable recall setting and large-scale deployment of systematised screening programs.
Study limitations
This research project has both strengths and limitations. Firstly, the data was collected from handwritten forms (See Appendix B in the Online Supplementary Document) and these forms were not always complete. Secondly, the VIA/VILI impression was not always adequately recorded for each women’s visit. The staff in the Women’s Health clinic included clinicians of varying medical qualifications, from third-year post-graduate medical students to qualified doctors and specialists in obstetrics and gynaecology. While VIA/VILI interpretation was overseen by a senior clinician experienced in the interpretation of results, this is an overall limitation of VIA/VILI in any clinical setting.
Future research implications
Exploring the utility and sustainability of POC testing in resource-deprived communities is paramount. Developing countries account for 85% of the global CC burden.25 An organised screening program depends on reliable clinical laboratories, systematised medical follow-up and access to appropriate care. POC testing offers a solution to resource-deprived settings. Laboratories are not required and a same-day result enables access to immediate appropriate care. POC testing is the solution to delayed diagnosis and loss to follow-up. More extensive studies looking at the prevalence of oncogenic HPV strains will be vital in designing suitable preventive care programs for CC. Policymakers and funders of vaccination programs look at research data that economically and clinically justify the investment. Future studies should determine the prevalence of different HPV strains on a larger scale in the Philippines.
It is recommended that future studies perform a cost analysis on the different screening methods for CC in low income and developing countries.
It would be beneficial to compare the cost of VIA/VILI screening and HPV testing with offshore processing. Additionally, future studies should compare the price of HPV testing with offshore processing to POC testing with instant referral.
Previous studies have demonstrated major barriers for CC screening in rural communities in general are: (i) inaccessibility26; and (ii) fear, embarrassment and anticipated shame.27 For the future, we recommend a qualitative study exploring the specific barriers preventing women from undergoing CC screening in this community. This would allow for optimisation of the program and greater uptake.
CONCLUSIONS
This study suggests HPV-PCR testing is superior at detecting HPV before cervical changes occur. Larger studies looking at the prevalence of oncogenic HPV strains will be vital in designing suitable preventive care programs for CC. Further research is imperative for policymakers and funders of vaccination programs. This is to ensure the best decisions are made for the community both economically and clinically. Future studies could perform HPV audits looking at data from across both rural and metropolitan settings in the Philippines. For this community in particular, qualitative studies to determine barriers in CC screening would enhance the number of patients captured and retained throughout the clinic.
Ethics statement
This research complies with the Australian National Health and Medical Research Council (NHMRC) National Statement on Ethical Conduct in Human Research. A Human Ethics Research Committee (HREC) approval was obtained (HREC reference: 016204S).
Funding
The research presented in the manuscript received funding from Notre Dame Australia Rural Clinical School, and the Team Philippines Project.
Authorship contributions
All four authors (Dr Elena Harty, Dr Samantha Carr, Zelda Doyle, and Prof Charlotte Hespe) made a substantial contribution to the conception, design of the work and the acquisition, analysis and interpretation of data for the work. Dr Elena Harty drafted the work, and Dr Samantha Carr, Zelda Doyle and Prof Charlotte Hespe reviewed it critically for important intellectual content. All authors reviewed the final submission piece. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure of interest
The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose no relevant interests.
Additional material
The article contains additional information as an Online Supplementary Document.
Correspondence to:
Elena Harty
Department of Medicine
University of Notre Dame Australia
160 Oxford St, Darlinghurst NSW 2010
[email protected]