Uninterrupted antiretroviral (ARV) supply for people living with HIV (PLHIV) is essential to maintain viral suppression and prevent HIV transmission. To continue providing essential services to PLHIV during the COVID-19 pandemic, providers and health systems needed to find ways to ensure people taking ARVs could access their medication consistently despite service provision challenges stemming from COVID-19 containment measures such as social distancing and travel restrictions. Service provision was further impeded by unintended impacts of COVID-19 such as health facilities avoidance by clients and supply chain shocks.1
COVID-19 presented an opportunity to scale up person-centric treatment approaches, specifically multi-month dispensing (MMD) and decentralized drug delivery (DDD), to maintain the continuity of HIV care while supporting safe access to essential medications.2,3 MMD is the provision of a three months or more of ARVs supplies instead of one to two months at each drug pick up. The literature reports that MMD keeps clients on treatment while saving them time and other opportunity costs associated with repeated visits to collect ARVs.2 DDD refers to ARV distribution through various channels and approaches within health facilities and in the community.4 MMD and DDD during COVID-19 have the additional benefits of decongesting health facilities and minimize potential COVID-19 exposure in facilities for both clients and staff and ensuring clinical resources are available for other essential care.4,5
African countries were among the least prepared to face the impact of the COVID-19 pandemic on the health system,6,7 threatening the quality and availability of HIV care for the 20.6 million PLHIV in the region.8 The Ending AIDS in West Africa (#EAWA) project is funded by the U.S. Agency for International Development (USAID) in Burkina Faso, and Togo (Box 1). Led by FHI 360, the project’s goal was to accelerate progress towards the 95-95-95 goals for PLHIV.9 As COVID-19 started to spread in Western African countries, the #EAWA project introduced strategies to ensure quality HIV prevention, care, and treatment in health facilities supported by the project in Burkina Faso and Togo.9,10
This paper describes COVID-19-related disruptions of ARV provision, and #EAWA’s strategies to maintain uninterrupted ARV access during COVID-19 in both countries. We then provide an assessment of MMD adaptations on the provision of ARVs.
METHODS
Setting and analysis population
Togo and Burkina Faso are two francophone Western African countries with low HIV prevalence and high disparities between key populations (KP) and the general PLHIV population. The HIV prevalence among adult PLHIVs is 0.6% in Burkina Faso and 1.9% in Togo.11,12 In comparison, sex workers have a prevalence of 6.8% and 13.2% while the prevalence among men who have sex with men is at 27.1% and 22.0%, respectively in both countries.12,13 In this context, the #EAWA project works with health facilities in rural and urban areas to improve preventive and curative services offered to KP and other PLHIVs. The analysis population for this evaluation is all clients who received HIV diagnosis services or ART between December 2019 and April 2021 in health facilities supported by #EAWA in both countries.
Data sources and data collection
Data on service disruptions due to COVID-19 and adaptations to address service provision challenges were gathered through written communications with #EAWA staff and from two briefs developed by the project. To assess how program and policy adaptations affected ARV services during the pandemic, we conducted a secondary analysis of routine facility-level programmatic data collected in the 17 #EAWA sites in Burkina Faso and the 25 in Togo between December 2019 and April 2021. All the programmatic data were electronically collected in health facilities and uploaded to a central server where we extracted them for analysis.
Data analysis
We conducted descriptive analysis of select indicators (see list below) in Excel. Results were stratified by rural/urban location and type of facilities (public or NGO) to assess any contextual differences. All data were aggregated at the facility level and did not include any individual level or personally identifiable information. Indicators examined included:
-
The number of expected ARV refills by month, defined as the number of clients who were given an appointment for ARV renewal during that month, not including those who died or transferred out.
-
The number of actual ARV refills by month, defined as the number of clients who received ARVs in the month among the clients expected for refills and those newly diagnosed with HIV who were enrolled on treatment.
-
Receipt of MMD, defined as clients who received enough ARV supplies to cover: (i) 3-5 months, or (ii) 6 months and more of treatment.
Ethical considerations
This evaluation received a non-human subjects’ research determination from the FHI 360 Protection of Human Subjects Committee (project # 1725659-1).
RESULTS
COVID-19 disruptions and adaptations at the government level
Burkina Faso and Togo reported their first COVID-19 cases in early March 2020. In reaction, both countries quickly declared a state of emergency to curtail the spread of COVID-19. Subsequently, between late March and early April 2020, the two countries enforced border closures, lockdowns, travel restrictions, and curfews. Within a month, these measures affected the provision of HIV care and treatment due to clients’ reduced ability to travel to health facilities and fuelled fear of health facilities being COVID-19 hotspots.9
In December 2019, before the pandemic, the National AIDS and STI Control Program (NACP) in Burkina Faso adopted MMD, allowing 3-5 months of ARVs to be distributed at a time to clinically stable clients[1]. This strategy was expanded in early 2020 to include 6-month MMD and community-based distribution of ARVs. Togo had a restrictive MMD policy pre-pandemic allowing only PLHIV with a suppressed viral load to be eligible for MMD. In March 2020 the Togo NACP modified HIV treatment protocols to ensure all HIV-positive clients, regardless of viral load, were eligible for 3-month supply of ARV (called “MMD for all”) but did not allow for 6-month MMD due to supply issues.
#EAWA’s approach to addressing the COVID-19 challenges and maintain ARV access
In April 2020, #EAWA adopted COVID-19 contingency plans for a continued provision of HIV care to clients in both countries. Under these plans, #EAWA continued implementing the national MMD policy adopted pre-pandemic in Burkina Faso, and rapidly implemented the “MMD for all” policy in Togo. In both countries, the project accelerated the implementation of DDD approaches expanding ARV provision to include facility-, community-, and home-based distribution of commodities. These options were designed to increase client confidence in accessing care despite concerns with visiting health facilities. In both countries, the project secured support from administrative authorities and law enforcement officers to guarantee transportation passes to conduct community-based activities during the lockdown.
To support clients preferring facility-based care, #EAWA Burkina Faso implemented formal appointments for health facility visits to maintain social distancing. In Togo, clients preferring facility-based care were provided reimbursement for transportation fees, and facilities were provided personal protective equipment, thermometers, and hand sanitizers to maintain a safe service provision environment.
The contingency plans also included measures to encourage adherence among PLHIV. In Burkina Faso, these measures included phone- and internet-based consultations. In Togo, adherence was encouraged via reminders sent by SMS or phone calls prior to refill appointments, reminder calls for those who missed appointments, and enhanced tracking of clients who experienced a treatment interruption. In parallel, the project conducted home and community visits to track unresponsive clients or those without cell phones.9,10
In both Togo and Burkina Faso #EAWA shifted reporting of ARV pick-ups, including the reporting on distribution of MMD, from monthly to weekly, to monitor how COVID-19 and the corresponding restrictions impacted ARV supplies. Each country team collected data on the quantity of pills dispensed, MMD dispensation, the location of dispensing (DDD), and the number of clients who were expected to pick up pills versus those who did.
Despite these adaptive measures, ARV provision remained challenging. In April 2020, Burkina Faso experienced ARV shortages due to global supply chain shocks. Similarly, Togo experienced limitations to ARV supplies due to border closures and lockdowns starting in May 2020, impacting particularly paediatric and second-line drug formulations first, and then all formulations. To prevent stockouts and treatment interruptions, both countries discontinued MMD and reverted to providing one or two months of ARVs, depending on ARV availability onsite. This approach allowed continuous delivery of ARV, with no stock outs, to all PLHIV served by #EAWA in both countries. As COVID-19 cases declined and restrictions eased, Burkina Faso and Togo’s ARV supplies were replenished by PEPFAR with 90-day pill packs in May 2020 and July 2020, respectively, which permitted 3-month MMD to restart.
Assessment of #EAWA’s adaptive MMD strategy on provision of ARV during the COVID-19 pandemic
Burkina Faso
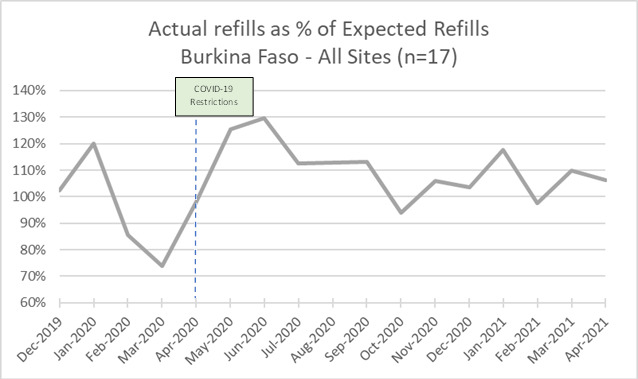
a) Expected versus actual refills
Between December 2019 and January 2020, the number of clients receiving ART refills exceeded the number of clients expected to refill their ART each month (Figure 1 shows that the proportion of actual refills relative to expected refills was above 100% during that period). Then, the number of actual refills was consistently lower than the number of expected refills between February and April 2020, with a dip in March when the first COVID-19 case was detected. This pattern was mostly reversed between May 2020 and February 2021 when there was more actual than expected refills, except in October 2020 and February 2021. Between May and October 2020, the data shows a 3-month pattern (May-July, August-October) of excess clients receiving refills compared to expected refills followed by a dip.
In both public (n=10) and NGO facilities (n=7) (data not shown), differences between expected and actual refills match the pattern for all sites, although NGO sites showed smaller differences between expected and actual refills. Disaggregation by rural (n=3) and urban (n=14) (data not shown) shows a larger difference between expected and actual refills in rural sites compared to the urban sites.
b) Dispensation of MMD
As the supply chain constricted after March 2020, the levels of MMD declined in Burkina Faso. The amount of 3–5-month MMD decreased by 17% in May 2020 compared to pre-pandemic levels (December 2019 – March 2020) while those receiving less than 3-month MMD increased during the same period (Figure 2). There was then a sharp increase of 3–5-month MMD from May to July, which then remained relatively steady, comparable to pre-pandemic levels. The proportion of clients receiving 6-month MMD continued to increase, more than doubling between July 2020 (13%) and March 2021 (33%). From December 2020 onward, over 90% of public site clients were receiving multi-month dispensing of ARVs.
Public and NGO sites had different patterns of MMD dispensation (data not shown). While public sites’ levels of MMD had a similar pattern to all #EAWA sites, the impact of COVID-19 on MMD was milder in NGO sites where 81% of clients received MMD in May 2020 compared to 53% in public sites in the same month. However, NGO sites reported lower rates of 6-month MMD compared to 3 -5month MMD starting in April 2020, in a reversal of pre-pandemic trends. A comparison between urban and rural sites shows that 6-month MMD was virtually non-existent in rural sites. Rural sites have fewer clients receiving MMD compared to urban.
Togo
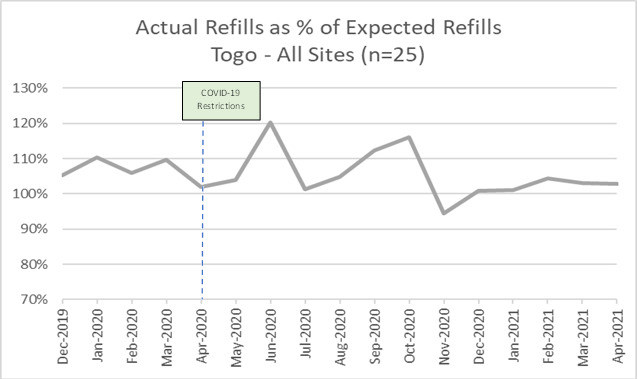
a) Expected versus actual refills
In Togo, the number of actual ARV refills exceeded expected refills every month from December 2019 – October 2020, with peaks in June and October 2020. In November 2020, the number of actual refills was slightly lower than the expected refills, a trend which was then reversed from December 2020 to April 2021 (Figure 3).
Similar patterns of expected and actual refills are seen in public (n=16) and NGO facilities (n=9), with reduced differences between expected and actual in NGO sites likely due to slightly lower total client volumes (data not shown). Starting in October 2020, a three-month pattern of increase (October – December 2020, January – March 2021) is again visible.
b) Dispensation of MMD
Between December 2019 and March 2020 (see Figure 4), 3–5-month MMD was being accessed by approximately one-third of clients prior to COVID-19 when it rapidly increased to approximately 60% in April and May 2020. In July, the share of 3-5-month MMD fell to approximately 15%, but then rebounded to approximately 60% by September, when MMD was reinstated, and reached a peak of approximately 76% in December 2020. The percentage of 6-month MMD remained in the single digits until March 2021, then reached approximately 20% in April 2021. By April 2021, 80% of #EAWA’s clients in Togo were on some version of MMD.
Looking at MMD by type of site (data not shown), we see a similar pattern in the public facilities as in the overall sample. Public sites also saw an increase in 6-plus month MMD in February 2021 reaching approximately 25% by April. NGO sites had a similar pattern overall with greater variability starting in the last three months of 2020 and slightly lower shares of six-month MMD.
DISCUSSION
Examining the number of expected versus actual refills offers a view of client demand for and ability to access treatment as well as a perspective on programmatic activities to identify new clients for treatment. Expected refills reflects the number of clients continuing treatment from month to month; it does not include those who have newly initiated treatment in that month. In Burkina Faso from February through April 2020 expected refills were consistently higher than actual refills showing clients were continuing care and the number of clients enrolled on treatment was growing but not all of those were actually getting their medicine monthly during this period. Both expected and actual refills dip in March 2020 as fewer people returned to get medications and fewer initiated treatment, reflecting the full impact of lockdowns. In May 2020 actual refills were higher than expected perhaps reflecting increased programmatic activity in identifying new clients, as well as clients who ran out of ARV during lockdowns returning for refills. Starting in May 2020, there is a 3-month pattern (May – July, August – October, November – January, and February – April) suggestive of clients on 3-month supply returning on schedule for refill. Data suggest rural sites were slower to recover from pandemic restrictions than urban in Burkina Faso, but NGO still were similar to those overall.
In Togo, the differences between expected and actual refills were less pronounced than Burkina Faso. There was similar growth in both the number of expected and actual refills between December 2019 and March 2020 showing programmatic growth, followed by a drop in both between April and June 2020 likely due to COVID-19 travel restrictions and clients’ inability or unwillingness to access health facilities. As with Burkina Faso, we see a post-lockdown surplus of actual over expected in June 2020 followed by a three-month pattern of increase suggestive of cohorts of clients on three-month MMD returning on schedule.
The varying number of clients receiving MMD reflects changes in dispensing policy and supply chains and the resulting programmatic adaptations to these factors. In Burkina Faso, pre-COVID policy allowed for up to five-month MMD; this was briefly expanded to six MMD in early 2020, but in April 2020 this was modified, and all clients started receiving one-month supply to ensure continued access to ARV to all without stockouts. This continued through May when Burkina Faso received restock from PEPFAR and MMD was able to restart. Data reflects this as both three- to five- and six-month MMD dips in May but then rebound sharply between May and July following resupply.
In Togo, the NACP modified treatment protocols in March 2020, creating “MMD for All” whereby all HIV-positive clients were eligible for three-month MMD, as opposed to only those with viral suppression in pre-pandemic. Levels of MMD increased rapidly to approximately 60% in May as a result of this policy change. Supply shortages in May then required the NACP to suspend MMD and shift to provision of one- to two-month supplies for all clients to ensure availability of treatment. This is reflected in the rates of MMD dropping to approximately 15% of clients receiving three- to five-month MMD in July. Following resupply of 90-day pill packs by PEPFAR in August, MMD again rebounded to approximately 60% in September and grew further to 76% in December 2020. Data show levels of MMD similar in public and NGO facilities with most clients receiving some MMD.
Early in the 2020 there was significant concern over the impact of the COVID-19 pandemic on the provision of HIV services in sub-Saharan Africa.14 Modeling efforts to predict the potential impact of disruptions to HIV service provision on HIV-related deaths found that disruptions to the supply of ART would have the largest impact of any potential COVID-19 disruption and identified ensuring an uninterrupted supply of ART as the priority for governments and donors to avoid additional HIV-related deaths. The impact of COVID-19 on HIV services was initially significant in Africa. In 2021, the WHO found the number of countries reporting disruptions to the provision of ART was higher in the African region compared to all others.15 COVID-19 triggered preventive measures and policies in Togo and Burkina Faso that initially disrupted service provision to PLHIV. The governments of both countries then acted rapidly to modify health policies related to HIV care to minimize the impact of COVID-19 measures. Similar results demonstrating the resilience of ART provision through the use of MMD, centralized ART pick up, and community-based service delivery have been documented in other African countries.16,17 A desk review of 21 PEPFAR-supported countries by Bailey et. al found that 16 of these countries (including the west African countries of Nigeria, Cameroon and Cote d’Ivoire), adapted MMD policies or promoted scale-up of MMD in response to COVID-19 with MMD coverage increasing from 49% pre-COVID to 72% in the second quarter of 2020.2 Similarly, facilities in Togo and Burkina Faso supported by the #EAWA project were able to adapt service delivery strategies in response to the constraints imposed by the pandemic to focus on ensuring provision of ART.
This assessment has some limitations readers should consider in interpreting the results. The generalizability of our findings is limited to #EAWA sites and not to all the HIV programs in Burkina Faso and Togo. Our results are descriptive and not intended to draw conclusions about associations between COVID-19 and ART services. Also, our dataset was not primarily collected for research purposes; therefore, there might be unmeasured factors other than COVID-19 that explain the variations in expected and actual refills and MMD dispensation reported in this assessment.
CONCLUSIONS
Examination of programmatic data from this HIV program shows that COVID-19 provided the impetus to embrace MMD to ensure continuity of care. As supply chains restricted, MMD had to be adapted, but once supplies stabilized, there was continued momentum for MMD. #EAWA’s rapid adoption of adaptive strategies to face commodities shortages and stockouts helped to maintain continuity of care to PLHIV despite COVID-19 restrictions in both countries. Additionally, the availability of and ability to access donor supplies in the form 90-day packs of drugs made the provision of MMD logistically easier. Overall, most clients were receiving 3-5 MMD in April 2021 with a growing use of 6+ month dispensing as conditions improved. Despite an extremely challenging and shifting environment, the #EAWA project was able to respond nimbly to ensure clients stayed on treatment. The initial fears of disastrous impact of COVID-19 on HIV service provision were instead a call to ensure continuity of services through adaptations to service delivery.
Disclaimer
This publication was made possible by the generous support of the American people through the United States Agency for International Development (USAID) and the United States President’s Emergency Plan for AIDS Relief (PEPFAR) through the #EAWA project, cooperative agreement number AID-624-A-17-00004. The contents of this publication are the sole responsibility of FHI 360 and do not necessarily reflect the views of USAID, PEPFAR, or the United States Government.
Data availability
Data supporting the findings of this study are available from the corresponding author (DB) on request.
Funding
The research presented in this article was funded by the United States Agency for International Development (#AID-624-A-17-00004).
Authorship contributions
Conceptualization: EE, DB, RH, JPT; Data acquisition: GY, GAK, JPT; Data analysis: RH, GY, GAK; Manuscript drafting: EE, DB, RH; Review of drafts: GY, GAK, JPT; Approval of final manuscript: EE, DB, GY, RH GAK, JPT.
Disclosure of interest
The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose no relevant interests.
Correspondence to:
Dieudonne Bidashimwa, MD, Ph.D.
FHI 360
359 Blackwell Street, Suite 200
Durham, NC 27701 USA
Email: [email protected]
Burkina Faso’s national HIV treatment guidelines define “clinically stable clients” as clients who have received ART for at least one year and who have no adverse drug reactions requiring regular monitoring, no disease, are not pregnant, are not breastfeeding, have a good understanding of lifelong treatment adherence and show evidence of treatment success (i.e., two consecutive viral load measurements below 1000 copies/ml). In the absence of viral load monitoring, an increase in CD4 cell count or a CD4 count above 200 cells/mm³ can be used to indicate treatment success.


.tif)

.tif)
.tif)
.tif)