The Sustainable Development Goals (SDGs) recognize child mortality as a major global health problem and aim to end preventable neonatal and child deaths by 2030.1 In 2020 alone, an estimated 5 million children under the age of 5 years died worldwide; of those, 2.4 million deaths occurred during the neonatal period, the first 28 days of life.2 The global neonatal mortality rate (NMR) is an estimated 17 deaths per 1,000 live births, with a disproportionately higher rate in sub-Saharan Africa (27 deaths per 1,000 live births).2 The leading causes of neonatal mortality are preterm birth (35%), intrapartum complications (24%), and infections (15%).3 SDG 3 includes targets to decrease NMR to 12 deaths per 1,000 live births and under-5 mortality rate (U5MR) to 25 deaths per 1,000 live births in all countries by the year 2030.1 Progress to achieve these targets has been notably uneven across countries and between regions, and the COVID-19 pandemic may likely reverse recent gains in child survival.2,4 Overall, only a few countries are on track to reach the NMR and U5MR targets by 2030. If current trends persist, two-thirds of countries in sub-Saharan Africa will miss the targets.5 Accelerated progress towards the unfinished SDG agenda requires new paradigms, strategic thinking, and innovative approaches to reduce child mortality, especially during the critical neonatal period.
Neonatal hypothermia, defined as a core body temperature less than 36.5°C, contributes to neonatal mortality.6–8 The World Health Organization (WHO) classifies hypothermia into three groups based on severity: mild hypothermia (36.0-36.4°C), moderate hypothermia (32.0-35.9°C), and severe hypothermia (<32.0°C).7 Regardless of gestational age and weight at birth, neonatal hypothermia increases the risk of death fivefold.9 For every degree Celsius drop in neonatal body temperature, the risk of mortality increases by 80%.10 While unresolved and untreated neonatal hypothermia is not a direct cause of mortality, it can increase the risk of developing comorbidities like hypoxia, sepsis, hypoglycemia, apnea, and poor weight gain – all of which increase the risk of mortality.11 Infants lose heat through radiation, evaporation, conduction, and convection, and it is vital to target these forms of energy loss to ensure newborns are kept warm.7 Thermal protection is recognized as a key component of essential newborn care. Effective strategies to prevent the development of neonatal hypothermia consist of a warm delivery room, wrapping and drying immediately after birth, skin-to-skin contact and kangaroo mother care (KMC), breastfeeding, delaying bathing and weighing the newborn, using appropriate clothing and bedding, keeping the newborn with the mother, ensuring warm transportation and resuscitation if necessary, and increasing awareness and training for neonatal hypothermia.7 Regular temperature monitoring further facilitates the early recognition and treatment of hypothermia. However, regular temperature monitoring in low-resource settings remains challenging due to understaffed and overcrowded wards.6,11 The lack of trained staff, expensive resources, poor provision and quality of care, and limited maternal education on newborn care further hinder the prevention, detection, and treatment of hypothermia.6,10 Consequently, neonatal hypothermia is poorly detected and remains an invisible public health challenge.
The prevalence of neonatal hypothermia varies by country, ranging from 11% to 95%, with the highest prevalence rates in sub-Saharan Africa.12,13 Differences in study design, study population, temperature measurement instrument and place, and contextual factors between countries may explain the variation in the prevalence of hypothermia. Understanding the prevalence and factors driving neonatal hypothermia in different local settings is important. Previous studies have identified factors associated with neonatal hypothermia, including neonatal factors (e.g. gestational age, sex, and birth weight), maternal factors (e.g. education, occupation, and marital status), and pregnancy-related factors (e.g. mode of delivery and location of delivery).12–17 Premature and LBW infants are at the highest risk for hypothermia, as they have less brown fat, less subcutaneous fat, and inefficient metabolic heat production.8,13
Despite being a significant risk factor for neonatal mortality, hypothermia is still understudied in low-resource settings, and there is a paucity of studies documenting the occurrence of neonatal hypothermia and identifying local risk factors.12–17 This study aimed to describe the distribution of neonatal hypothermia and examine the risk factors associated with neonatal hypothermia in LBW infants in Accra, Ghana. Ghana has an estimated neonatal mortality rate of 23.1 deaths per 1,000 live births and 14% of babies are born LBW.18
METHODS
Study design and setting
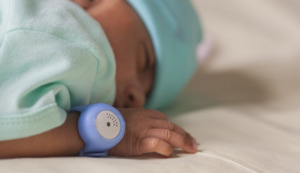
The present study analyzed primary data collected in a study assessing the accuracy of the BEMPU® TempWatch (BEMPU Health, Bangalore, Karnataka, India), a continuous temperature monitoring bracelet used to detect neonatal hypothermia. The BEMPU® TempWatch consists of a silicone band with a thermistor metal cup that emits an audio alarm and flashes orange when body temperature drops below 36.5°C (Figure 1).19 The bracelet fits newborns up to 3,500 grams. The effectiveness of the BEMPU® TempWatch as a tool to alert caregivers of neonatal hypothermia has been assessed in several studies. The sensitivity and specificity of the TempWatch in detecting hypothermia were 98.6% and 95%, respectively.20 Emerging evidence suggests that the device improves early recognition of hypothermia and may increase weight gain and promote KMC.19–23 Nevertheless, further validation of the device is warranted.23
The present study was conducted at Korle Bu Teaching Hospital in Accra, Ghana. The hospital is the largest referral center in West Africa, with a capacity of over 2,000 beds and an average attendance of 1,500 patients a day. The Neonatal Intensive Care Unit (NICU) of the Korle- Bu Teaching Hospital (KBTH) provides care for premature and critically ill term babies and has a nominal capacity of 60 cots, warming platforms and incubators, though this number is often exceeded. The unit receives referrals from health facilities in the country’s southern half. From 2011 to 2015, the mean number of admissions was 1,843 per annum, and the overall mortality rate was 19.2%.24 Typically, infants admitted to the NICU are triaged based on their weight, with infants less than 2,000 grams kept in incubators with minimal clothing. Monitors attached to the incubators display the air temperature in the incubator and are adjusted as necessary. Clinically stable infants weighing 2,000 grams are kept in cots and clothed and wrapped. Mothers of infants are permitted in the NICU at designated infant feeding times to express breastmilk or directly breastfeed their infants. An attached KMC room has space for up to 6 mother-infant dyads.
Infants were eligible to participate in the study if they met the inclusion criteria: less than 28 days old, admitted to the NICU, LBW, and clinically stable. Infants whose mother was less than 18 years old or expected to be discharged from the NICU within 24 hours were excluded. Potentially eligible infants were identified by local study nurses who then approached their mothers to determine interest in participating in the validation study. The study was fully explained to all interested mothers. Written informed consent to participate in research was sought from mothers of eligible infants before collecting data. The required sample size of 255 was determined using a one-sample equivalence test for proportions with an equivalence threshold set at 15 percentage points and assuming a sensitivity of 90%, a power of 80%, a type-I error rate of 5%, and a loss to follow-up of 15%.
After obtaining informed consent, study nurses administered a standardized questionnaire to the mothers to collect sociodemographic data on education level, occupation, marital status, religion, and ethnicity. The questionnaire also collected data on maternal reproductive and health history (gravida, parity, antenatal care (ANC), pregnancy complications, mode of delivery, and type of birth) and post-natal history (gender of the infant, birth weight, APGAR scores at 1 and 5 minutes after birth). After completion of the questionnaire, study nurses applied the BEMPU® TempWatch to the wrists of the infants and recorded baseline information on thermal care practices; specifically, whether the baby was wrapped or covered in a blanket or cloth, wearing a hat or cap, wearing a diaper, wearing socks, wearing mittens or gloves, and being treated in an incubator. The study nurses then recorded the axillary temperatures of the infants every 4 hours using digital thermometers over a 24-hour monitoring period. Digital thermometers have an accuracy of about 0.02°C when used for measurement from the axillary site.25 Whenever the alarm on the BEMPU® TempWatch sounded, indicating an episode of hypothermia, the study nurses measured the infant’s axillary temperature, again documented thermal care practices, and recorded any clinical actions taken. Each infant had at least seven temperature readings over the 24-hour monitoring period. Data were collected and stored on Android tablets using Open Data Kit (ODK) software and later uploaded to a secure server.
Statistical analysis
The primary outcome for this analysis was axillary temperature measured by a digital thermometer. Independent variables examined were gravida, parity, singleton or multiple births, mode of delivery (vaginal or cesarean section), infant sex, age, birth weight, gestational age at birth, Apgar scores at 1 and 5 minutes, current feeding, and thermal practices at the time of birth and at the time of temperature recording. Univariate and multivariate linear regressions were performed to examine associations between temperature and various independent variables. Due to multiple observations of each infant, we used generalized estimating equations (GEE). Univariate associations with P<0.1 were included in the initial multivariate model. A forwards stepwise procedure with inclusion criteria of 0.05 was used to select the final multivariate model. The statistical significance level was set at P<0.05. Temperature changes, confidence intervals and p-values were reported for each independent variable. All statistical analyses were performed using STATA 17.0 (StataCorp LLC, College Station, TX, USA).
Ethics consideration
Ethical approval for human subjects research was obtained from the Institutional Review Boards (IRB) at the University of South Carolina (Pro 00095600) and Korle Bu Teaching Hospital (KBTH-IRB/00052/2020). Written informed consent to participate in research was obtained from mothers of infants in a local language or English prior to enrolment in the study.
RESULTS
Between May 2021 and January 2022, 255 eligible infants at the Korle Bu Teaching Hospital were enrolled and monitored for 24 hours. Due to missing temperature data, one infant was excluded from the present analysis. Of the 254 infants included, most were preterm (92.9%), were delivered by C-section (53.9%), were exclusively breastfeeding (70.5%), and had an APGAR score ≤6 at 1 minute after birth (69.2%) (Table 1). Less than half of infants were male (42.1%), VLBW (< 1,500 grams) (49.6%), part of multiple births (24.0%), extremely preterm (<28 weeks gestation, 8.3%) and seven days old or less at enrollment (47.6%). Most infant mothers were older than 25 years (71.7%), were married (64.8%), had completed secondary education or higher (71.3%), attended at least one ANC visit during pregnancy (94.5%), were multiparous (52.6%), and were multigravida (65.3%).
Of the 1,948 axillary temperature readings obtained from the 254 infants, 44.5% were hypothermic (<36.5°C). 26.5% of temperature readings met the criteria for mild hypothermia (36.0-36.4°C), while 18.0% met the criteria for moderate hypothermia (32.0-35.9°C). None of the readings met the criteria for severe hypothermia (<32.0°C). Overall, mild and moderate hypothermia were prevalent in this population, with 85.8% of infants having at least one temperature reading less than 36.5°C during the 24-hour monitoring period. The incidence of hypothermia was higher among VLBW infants (86.3%) and extremely preterm infants (100%).
Univariate linear regression models identified factors significantly associated with neonatal temperature (Table 2). Being part of multiple births, being VLBW and having a lower gestational age at birth were associated with lower temperatures, while primiparity was associated with higher temperatures, on average. Of the thermal practices considered, only skin-to-skin contact, being wrapped at the time of temperature recording, wearing a diaper at the time of temperature recording and being treated in an incubator at the time of temperature reading were protective against lower temperatures (P<0.05). There were no significant associations between temperature and wearing a hat/cap, socks or gloves/mittens (P>0.05). After adjusting for significant covariates, the multivariate linear regression model demonstrated that compared to exclusively breastfed infants, mixed-fed (formula and breastmilk) infants had temperatures lower by 1.08°C, and formula-fed infants had temperatures that were higher by 0.38°C. VLBW infants had lower average temperatures compared to infants who were not VLBW at birth (β=-0.14°C), while extremely preterm infants had lower temperatures than term infants (β=-0.70°C). Infants who had skin-to-skin contact at birth were wrapped and treated in an incubator had higher temperatures (0.20°C, 0.36°C and 0.67̊°C respectively).
DISCUSSION
This study sought to describe the prevalence of neonatal hypothermia and associated risk factors in Accra, Ghana. Several of the study findings have implications for the thermal care of LBW infants in low-resource settings. First, 85.8% of infants experienced hypothermia at least once during the 24-hour monitoring period. This prevalence rate lies within the range previously described in other settings (8.1% to 94.9%).13 However, the prevalence rate lies at the higher end of the range, most likely because being LBW was part of the inclusion criteria for this study. About half (49.6%) of infants in this study were VLBW, and most (92.9%) were preterm. Of the VLBW infants, 86.3% had a hypothermic episode during the 24-hour monitoring period; of the extremely preterm infants, all (100%) had a hypothermic episode during the 24-hour monitoring period. After adjusting for other covariates, including incubator use, there was a 0.11°C difference in neonatal temperature between VLBW and other infants and a 0.73°C difference between extremely preterm and term infants. These findings align with previous studies that have indicated a higher occurrence of hypothermia among LBW infants and preterm infants.12–17
The study also highlights the importance of thermal care practices for preventing and managing neonatal hypothermia. Unsurprisingly, infants who had skin-to-skin contact at birth were wrapped and were in an incubator at the time of temperature reading had higher temperatures. Additionally, mixed-fed infants had significantly lower temperatures compared to exclusively breastfed infants. Underfunded health systems, shortages of human resources for health, lack of reliable electricity and limited financial resources have hindered the use of incubators and other technologies in low-resource settings.26 However, skin-to-skin contact is widely recognized as an inexpensive, effective and safe way to achieve and maintain stable neonatal temperature and prevent hypothermia, especially among clinically stable LBW and preterm infants in low-resource settings.27 Kangaroo mother care (KMC) which includes early, continuous, and prolonged skin-to-skin contact and breastfeeding, has been shown to reduce hypothermia, the incidence of infections, and the risk of mortality, while promoting weight gain and nurturing parent-infant attachment.28–30 Despite calls for universal coverage of KMC for LBW and preterm infants across the facility-community continuum, the implementation and scale-up of KMC has been slow due to structural, economic, logistic, and social barriers.31 Progress should be made to scale-up KMC and interventions that complement KMC, such as continuous temperature monitoring.
This study has several limitations. As a result of the study, thermal care practices may have been affected. The monitoring period was 24 hours, with no further information on infants beyond that period. Since the study only included LBW infants admitted to the NICU of one tertiary hospital, the findings may not be generalizable to other health facilities and infants in community settings.
CONCLUSIONS
Despite the limitations, the high burden of neonatal hypothermia calls for the prioritization of this invisible public health problem. Future studies should evaluate thermal protection education for nurses and mothers in facilities in low-resource countries to increase awareness and decrease the incidence of hypothermia. Studies should also evaluate the increased use of skin-to-skin contact to augment the thermal protection of LBW infants. Health providers must recognize hypothermia in LBW infants as an invisible public health problem and work towards optimal thermal management.
Acknowledgements
The authors would like to thank the staff members at the Korle-Bu Teaching Hospital and the mothers and neonates included in the study.
Funding
This work was supported by the Thrasher Research Fund (Grant 15194).
Authorship contributions
JP performed the data analysis and drafted the manuscript. MK conceived the study design, acquired the funding, coordinated the study, performed the data analysis and drafted the manuscript. BAD, PGO, and KSS conceived the study design, coordinated the study, collected and revised the manuscript. AS, ISM and RBD conceived the study design and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors completed the Unified Competing Interest form at http://www.icmje.org/disclosure-of-interest/ (available upon request from the corresponding author) and declare no conflicts of interest.
Correspondence to:
Mufaro Kanyangarara, Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208. Email: [email protected]