During the last several decades the health care infrastructure in Iraq has been seriously compromised as a result of war, economic sanctions, and political violence.1 During the 1970s, Iraq had one of the more robust health care systems in the Middle East, with 172 hospitals and 1,200 primary care clinics that provided free services through a public single-payer financing mechanism.2 However, the first Gulf War (1980-1988), i.e. the Iran-Iraq war, ushered in a long period of upheaval that significantly undermined health services. The second Gulf War occurred in 1991 and was followed by 13 years of United Nations and United States imposed economic sanctions further crippling the health care infrastructure and resulting in sharp increases in child morbidity and mortality related to not only a weakened health care services but also a significant burden of malnutrition as well as long-term damages to the water and sanitation systems.3 Chronic instability that followed the third Gulf War in 2003 led to the loss of central government control over most parts of Iraq, creating a sense of political insecurity and a high level of chronic stress, which can have a disproportionate effect on children. The state of conflict has also directly weakened the Iraqi health system: damaging government health care institutions, fracturing supply chains, and contributing to “brain drain” of key medical personnel.
Health care infrastructure in Iraq today is a shadow of what it was in the 1970s before war and economic sanctions. Due to violence and the deterioration of health services in Iraq, there are now only 7.8 physicians and 14.9 nurses per 10,000 population.4 This is 60% lower than the average physician-to-population ratio, and 35% lower than the average nurse-to-population ratio for the Eastern Mediterranean Region of the WHO.5 Of the 34,000 physicians in the country before 2003, the Brookings Institution has reported that approximately 2,000 have been murdered, 250 kidnapped, and 20,000 emigrated from the country. An estimated 70% of medical specialists that were in Iraq as of 2003 have since left the country.5
The broader challenges of the health care system in Iraq are experienced at all levels, including tertiary care settings where specialized services are offered. Pediatric cancer care is one aspect of health services that has been negatively impacted by limited resources in Iraq. The Children’s Welfare Teaching Hospital (CWTH) is one of the two central referral facilities for children with cancer in Iraq. It is a governmental hospital within the Medical City in Baghdad, which is a teaching medical complex for Baghdad College of Medicine. The pediatric oncology unit offers free (government-funded) care and treats all patients referred from a broad range of providers throughout Baghdad (40% of patient population) as well as other provinces in the country (60% of patient population), with the exception of Basrah and Kurdistan. Despite the fact that it is a tertiary center, CWTH suffers from persistent limitations in access to medications and supplies for cancer treatment. In addition to material constraints, its personnel must grapple with limited access to clinical training and medical literature. For example, management of brain tumors was not feasible until recently due to limitations in training and resources.
In high resource environments, recent years have seen significant progress in the survival of pediatric cancer patients. Five-year survival rates in the United States and France have increased from approximately 30% in the 1960s to greater than 80% more recently.6,7 In countries with limited resources, however, survival rates of childhood cancer are 20-30% lower than higher income settings. In a study among children with cancer in Iraq, the estimated 5-year survival rate was estimated at only 50%.8 Reduced survival in Iraq and other resource-limited countries may be in part related to delay in cancer diagnosis. Therefore, the present study aims to: 1) estimate the median time from initial symptom presentation to diagnosis of childhood cancer at the CWTH in Iraq; and 2) examine sociodemographic, economic, and clinical factors associated with delay in diagnosis in this vulnerable population.
Methods
This descriptive study included a cohort of 346 children 1 to 14 years of age that presented at the CWTH with newly diagnosed cancer. All children that presented consecutively for cancer care at CWTH between 1 January and 31 December 2012 were included in the study. Structured interviews were conducted with a primary caregiver within 7 days of admission. Some clinical characteristics and sociodemographic information were provided by the caregivers, including information on parents’ education and employment; location of residence; family size; number of children; father’s vital status; length of delay in cancer diagnosis; number of doctor visits prior to CWTH admission; diagnosis prior to cancer diagnosis; and treatment prior to diagnosis. Additional data were collected systematically from medical records and included data on the type of cancer; stage of solid tumors; site of tumor; cancer-related symptoms; and survival status within 30 days of admission.
Delay in cancer diagnosis was defined for total, patient, and physician delay. The total delay in cancer diagnosis was defined as the number of days between the onset of symptoms and the cancer diagnosis. The patient delay was defined as the time interval between the onset of symptoms until the visit with the first physician. Physician delay was defined as the time elapsed between the first contact with the health care system and the cancer diagnosis. These definitions are consistent with what was used by Dang-Tan and colleagues.9 For each of these delays, the median, mean, and range were calculated. Frequencies/ percentages were estimated for categorical variables. To examine the differences in the mean number of days of delay (total, patient, and physician delay) for family/child as well as physician/clinical characteristics a t-test was used for variables with 2 categories and analysis of variance was used for variables with more than 2 categories. For variables that were not normally distributed the Mann-Whitney or Kruskal-Wallis statistical tests were used. Statistical analyses were performed using SPSS, version 13. The study protocol was approved by the institutional review board of the Children’s Welfare Teaching Hospital, reflecting ethical approval for the study. There was no external funding available for the study.
Results
A majority of the 346 newly diagnosed patients admitted to the oncology unit at CWTH were five years of age or younger (57.2%) and over 59% were boys. The largest percentage of patients came from Baghdad governorate (43.6%), although over half came from other governorates, traveling as far as Basra in the south (Table 1). Approximately two-thirds of the patients came from rural areas. Median travel time to CWTH for families was 2 hours (range: 15 minutes to 8 hours). The family size ranged from 2 to 18 members, with the number of children ranging from 1 to 14. Mothers were 16 to 57 years of age, with nearly 38% having completed secondary school or a higher level of education. Approximately 16% of mothers were not literate. A large majority of the mothers primarily worked at home, caring for their children and families (91.0%). Fathers were 19 to 65 years of age and had a higher level of education with over 55% having completed secondary school or greater. Nearly 36% of fathers were employed and 60.4% were self-employed.
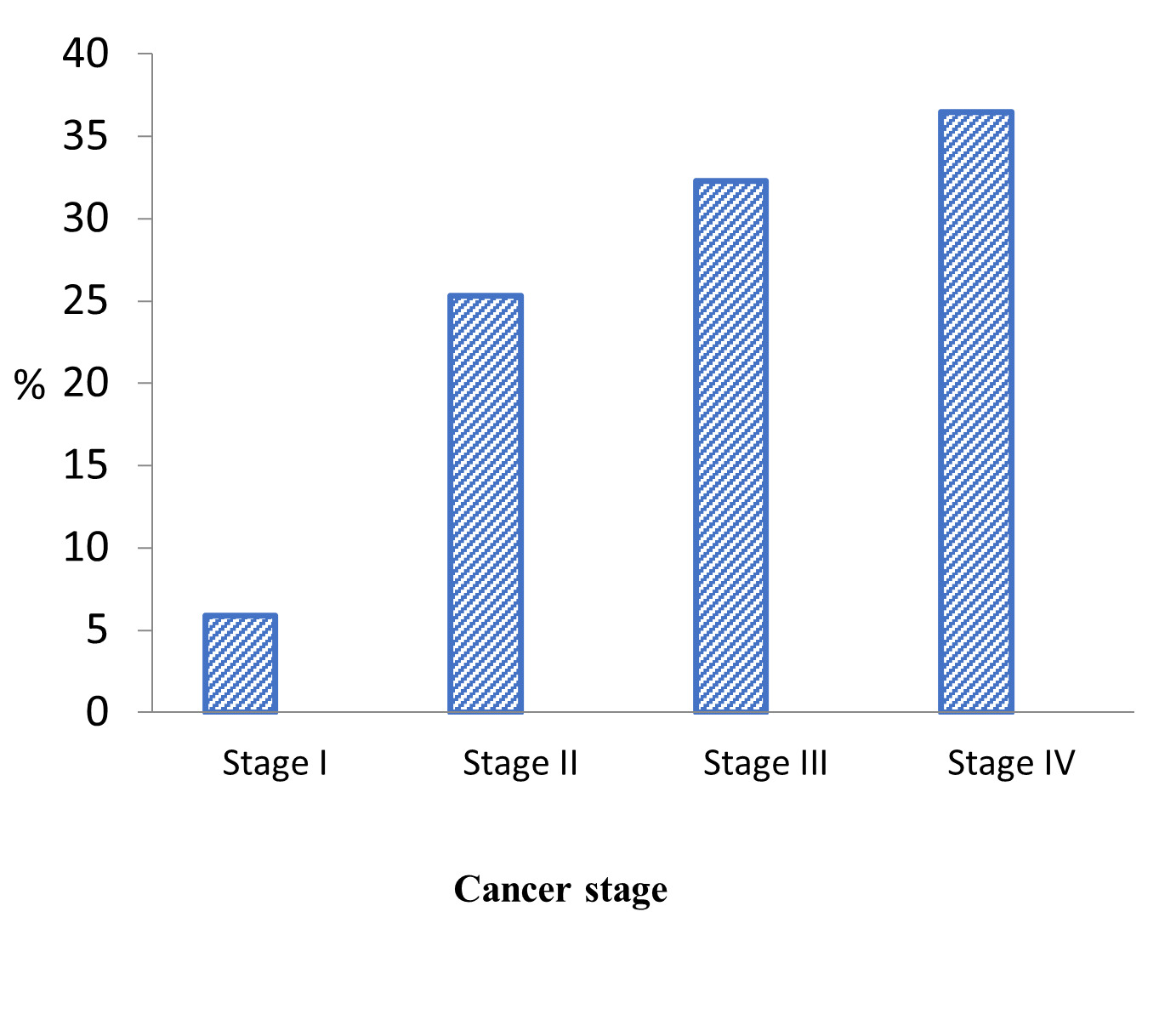
All types of childhood malignancies were eligible for treatment at the unit, with the exception of brain tumors. Leukemias represented the largest percentage of cancers presented at the unit (45.0%), followed by blastomas (23.6%), and lymphomas (18.8%). The most prevalent diagnosis was acute lymphoblastic leukemia (30.6%), followed by Non-Hodgkin’s Lymphoma (13.0%), and neuroblastoma (9.8%). Overall, the median number of days for diagnostic delay was 55, ranging from 3 to 1,093 days, nearly 3 years (Table 2). This diagnostic delay resulted in a large majority of patients (68.8%) with solid tumors presenting at Stage III (32.3%) or Stage IV (36.5%) cancer (Figure 1).
Among children with available treatment history prior to referral, nearly three-quarters were treated with antibiotics, followed by blood transfusion (8.3%), tonics (6.1%), and steroids (5.0%) (Table 3). This is consistent with infection being the predominant misdiagnosis (59.9%), followed by anemia (14.1%) and myalgia (5.8%). Initial misdiagnosis was one of the key factors leading to delayed cancer diagnosis; this is reflected in the finding that having more than three doctor visits prior to referral significantly delayed the children’s cancer diagnoses. This is also consistent with the observation that the median number of days for physician delay was much greater than what was observed for patient delay (43 versus 4 days) (Table 2). Overall, 12.7% of patients died within 30 days of admission. Among the patients that died, 58% had leukemia and 50% with a solid tumor presented as Stage IV.
A number of patient and family characteristics were associated with delay in cancer diagnosis including sex, age, family size, number of children, mother’s education, and father’s death. In particular, children 5 years of age or younger had a shorter delay in diagnosis compared to older children (84 versus 125 days, P=0.03). Those with a smaller family size and families with a single child also demonstrated a shorter delay in diagnosis. Mother’s education was protective— women who had post-secondary school attendance had a lower mean number of days of delay compared to women with less education (64 versus 106 days, P=0.003). Father’s death significantly delayed cancer diagnosis by nearly a year (289 days) compared with 97 days for fathers that were alive (P=0.001). For patient-related delay, similar to the total delay younger age and being a single child resulted in less of a delay for cancer diagnosis. In contrast, the father’s employment status was related to patient delay in diagnosis, whereby children whose fathers were employed had a longer delay in diagnosis compared to fathers that were not employed (P=0.006). Similar to total treatment delay, physician-related delay was also associated with sex and family size, with girls (70 versus 103 days, P=0.03) and children from smaller families (76 versus 108 days, P=0.001) experiencing less of a delay. Father’s death was also a risk factor, whereas mother’s education was a protective factor with respect to physician delay in diagnosis of childhood cancer (Table 4).
Physician and clinical characteristics were also associated with delay in diagnosis. In particular, the number of doctor visits prior to attending CWTH, type of cancer, and site of tumor were associated with total delay. Children with more than 3 doctor visits demonstrated a median delay of 120 days versus 78 days for children with 3 or fewer visits (P=0.001). Children with tumors in the head/neck or extremities also experienced a longer delay, 221 and 282 days, respectively, compared with those with tumors in the chest/back (195 days) or abdomen/pelvis (88 days) (P=0.001). For patient delay, children with advanced tumors demonstrated a shorter delay compared to those with early stage tumors (8 versus 29 days, P=0.003). A similar finding for patient delay was generally observed for tumor site with chest (12 days) and abdomen (10 days) sites demonstrating a shorter delay compared with tumors in the extremities (71 days), although tumors in the head and neck indicated a shorter patient delay to diagnosis (8 days) (P=0.001). Similar to total delay in diagnosis, longer physician delay was associated with more than 3 doctor visits (P=0.001) and tumor site in the head/neck or extremities (P=0.001) (Table 4).
Discussion
This is the first study to date that examines the delay in diagnosis in childhood cancer in Iraq. Delay in diagnosis in Iraq was significant, with a median total number of days in delay of diagnosis of 55, extending as long as three years. Reflective of delays in access to care is also demonstrated by the fact that over two-thirds of the children with tumors presented with Stage III or IV cancer and approximately 13% died within the first 30 days of admission. Late presentation of childhood cancer has also been associated with an increased risk of mortality in other resource-limited settings.10 Delay in presentation was often related to initial misdiagnosis by physicians prior to children receiving services at CWTH. In the present study, the delay in diagnosis was largely due to physician delay with a median of 43 days to cancer diagnosis. This was also reflected in the finding that multiple visits to doctors (greater than 3) were related to a longer delay in diagnosis— median of 120 days of delay in contrast to 78 days among children who had 3 or fewer doctor visits. In contrast, the median patient delay of cancer diagnosis was 4 days in the current study, although it ranged from 1 to 365 days.
Studies in higher resource settings have demonstrated a lower median number of total days of delayed diagnosis of childhood cancer. A study in Singapore reported a median number of total days of delayed diagnosis of 37,11 whereas studies in Canada and South Africa both reported a median of 34 days.9,12 Regionally, other countries in the Middle East generally demonstrated a lower median in number of days of delayed diagnosis ranging from 30 days in Iran to 47 days in Egypt.13–15 In the more resource-limited setting of Indonesia the median total number of days of delay was as high as 70.16 Countries in sub-Saharan Africa demonstrated the longest delays in diagnosis, ranging from a median of 85 days in Kenya to 111 days in Nigeria.17–20
The finding of this study that delay in diagnosis was largely related to physician delay has been observed by studies in other settings. For example, the median physician delay was 20 days in South Africa12 and 30 days in Canada.9 In Egypt, physician or diagnostic delay was 28 and patient delay was 3 and 8 days for two different studies.14,15 In Indonesia, the median delay related to the health care system was higher, at 49 days as compared to patient delay which was 5 days.16 In contrast, a later study in Singapore reported a much lower median physician delay of approximately 8 days.11 Compared to physician delay, median patient delay was lower for South Africa and Indonesia (median – 5 days),12,15 and Canada (median – 9 days),9 similar to findings from the current study; however, the median patient delay was higher in Singapore, reported as 21 days.11 Median patient delay was also higher in several studies in sub-Saharan Africa as compared to physician delay.17,19 In contrast, physician delay remained higher than patient delay in Ibadan, Nigeria (median of 62 versus 14 days of delay) and in western Kenya (median of 87 versus 4 median days of delay).18,20 In resource-limited settings, physician delay can be related to the high burden of infectious disease among children in these settings. This is reflected in the present study, in which the most prevalent treatment offered before presenting at CWTH was antibiotics. It may also be related to lack of awareness of childhood cancer as well as limited access to formal training in pediatric cancer in these settings, which is also the case in this local context of Iraq. The limitations in the overall health system in Iraq, including lack of adequate medical training in general, poor diagnostic facilities, as well as a low doctor: patient ratio provide a context that contributes to not only delays in access to cancer care but also reduced survival.8
A number of sociodemographic factors were associated with delay in diagnosis of childhood cancer in the present study, including age, sex, family size, number of children, mother’s education, father’s death, and father’s employment. Similar to the present study, child’s age was associated with delay in diagnosis in a number of studies,9,11,13,14,14,21,22 with older children generally experiencing a delayed diagnosis. This may possibly be a function of parents’ monitoring symptoms more closely in younger children. Alternatively, older children may be reluctant to share information about their symptoms if they think that their parents may not believe them. In addition, smaller organ size in younger children may result in faster progression of symptoms, alerting caregivers earlier.23 In contrast, sex was typically not associated with delays in cancer diagnosis in other settings,9,12,21,24 with the exception of a study in Denmark where girls experienced a longer delay.22 In addition, mother’s education demonstrated a protective effect, which was consistent with findings from a study in Israel where children of mothers with academic professions experienced a shorter delay in diagnosis compared to mothers who worked at home or in a manual occupation.25 Similarly, lower parental education and socioeconomic status were also associated with delay in cancer diagnosis among children in Egypt.14 In contrast, for the present study father’s employment had a negative effect on delay in diagnosis. However, this may be consistent with the finding that the father’s death was also associated with delay in cancer diagnosis, suggesting that fathers that are available may help to prevent diagnostic delays.
Several clinical and physician characteristics were associated with delay in childhood cancer diagnosis in this population, including the type and site of cancer, and the number of physician visits prior to presentation at CWTH. The greater number of doctor visits may be related to the non-specificity of symptoms and the relatively low incidence of childhood cancer. In the present study, childhood cancer was initially misdiagnosed primarily as infection or anemia, which was consistent with a study from South Africa, where infection was also the most common initial diagnosis.12 Similar to the findings from the present study, cancer and tumor type have been associated with delay in diagnosis of childhood cancer in other settings.9,13,14,17,21,24
There are a number of limitations in the study. Although the data were collected systematically, they are ultimately dependent on parents’ memory. This may have led to random misclassification, biasing results towards the null. However, this was possibly offset by the fact that some clinical outcome data were abstracted from medical charts. Similar to other resource limited settings, although health care is funded by the government, the out-of-pocket cost of transportation and the opportunity costs of time away from work or caring for other children at home may be prohibitive for some families, so it is likely that children in these circumstances may have significant delays but were unable to continue to pursue care and did not present at CWTH. Therefore, it is likely that these delays in diagnosis are underestimates. Additionally, the findings cannot be generalized beyond CWTH; however, since the unit is a main tertiary center for pediatric cancer care in Iraq it is likely that other centers are experiencing greater challenges and may potentially have worse outcomes.
Conclusions
Despite tremendous global advances in childhood cancer treatment outcomes in recent years, disparities in survival rates between high-resource and low- and middle-income countries persist.26,27 The situation at CWTH in Iraq reflects these broader disparities. Iraq’s decades of war and violence have contributed to the impoverishment of its people and the degradation of its health care system. This has heavily impacted the most vulnerable Iraqis, including children with life-threatening conditions such as cancer. Economic disparities and persistent violence have impeded access to care, especially for key technology-intensive medical equipment for radiation and other procedures; produced shortages or stock-outs of medications; resulted in low doctor-patient ratios; and limited training in pediatric oncology services. These trends are similar to other resource-limited settings.28 Iraqi pediatric cancer patients must contend with frequent misdiagnosis, delay in access to effective cancer care, and increased risk of mortality.10 The consequence has been premature death for thousands of children in Iraq since the advent of more effective treatment of childhood cancer.
Delayed access to life-saving care for childhood cancer will continue in Iraq unless appropriate action is taken at local, national and international levels. At the local level, it is critical that primary health care providers are sensitized to the problem of childhood cancer and given training on early identification of the key signs and symptoms, since physician misdiagnosis resulted in a significant delay in access to care.
Improvements must also be made in referral systems to reduce delays in access to care. Since childhood cancer is relatively rare and the symptoms are typically non-specific, structured treatment protocols and checklists can be developed to aid local providers in sustaining activities that are outlined in training sessions. However, increasing the availability of training and clinical protocols may only have a marginal impact if access to pediatric cancer care is not improved through action at national and international levels.
In order to improve access to quality care for childhood cancer in Iraq, national investment in the health care system must be increased. However, decades of war and sanctions have led to widespread impoverishment and declines in tax revenue, resulting in sharp limitations on available public funds. Therefore, international support of material and human resources to ensure adequate access to quality cancer care for children in Iraq will continue to be a necessity.
While Iraq is unusual in the direct impact of external intervention on its health care system in recent decades, limitations in access to care and treatment for childhood cancer are typical of many other resource-limited countries. Recently, there has been discussion of a UN “trust fund” to support care for non-communicable diseases in low- and middle-income countries.29 Findings from the present study illustrate the urgent importance of this initiative and others like it to ensure access to life-saving treatment for children suffering from cancer in Iraq and other resource-limited settings.
Acknowledgements
The authors would like to offer a special thank you to all of the patients and their families that participated in the study. In addition, we would like to extend our sincere appreciation for all staff that helped to make this study possible. We would also like to extend our gratitude to Maureen A. Smith, RN, who carefully reviewed the literature in this manuscript.
Funding
No external funding was available for this study.
Authorship contributions
WY, MFA, SAF, HHG, and SAA developed the study, were involved with the data collection and analysis, and drafted the manuscript. AS, MNK, and MCSF reviewed the manuscript and drafted sections that provided substantive comments. All authors reviewed and approved the final manuscript.
Competing interests
The authors completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available from request from the corresponding author), and declare no conflicts of interest.
Corresponding author
Mazin Faisal Al-Jadiry, MD
Children’s Welfare Teaching Hospital, College of Medicine-University of Baghdad
Department of Pediatric Hematology Oncology
Medical City, Baghdad, Iraq
[email protected]