Coronavirus disease 2019 (COVID-19), an infection caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in December 2019, in Wuhan, province of Hubei in China. Since then, it has become one of the world’s toughest health problems. The World Health Organization (WHO) announced on January 30, 2020 that the COVID-19 epidemic was a public health emergency of international concern and on March 20, 2020, that it was a pandemic.1 As of January 24, 2021, more than 97 million cases of COVID-19 have been reported worldwide, causing more than 2 million deaths.2 In just a few months, huge efforts have been made and several vaccines are in development.
This review summarizes COVID-19 vaccines clinical trials in phase III and IV and all published clinical trials’ results.
METHODS
A review of the scientific literature was conducted with the aim to study the therapeutic trials of vaccines against the coronavirus disease 2019 (COVID-19). To this end, as of January 24, 2021 a search for articles published on PubMed using the Mesh word “COVID-19 Vaccine”, as well as a search for protocols registered on ClinicalTrials.gov by combining the words “COVID-19”, “SARS-CoV-2” and “Vaccine” was made. The published WHO reports on candidate COVID-19 vaccines were reviewed. At first, citations that were not in French or in English and not a clinical trial or randomized controlled trial were excluded. Then, those with irrelevant information or subject and studies with focus other than COVID-19 vaccine development or clinical trial were excluded. For registered protocols, those in relation with drugs were excluded.
RESULTS
Included citations in the COVID-19 vaccine therapeutic trials review
On PubMed, 1227 articles were initially found, out of which 16 articles were selected. In the end, 14 articles were selected. On ClinicalTrials.gov, as of January 24, 2021, 72 phase III or IV, clinical trial protocols, encompassing vaccines and treatments have been identified initially, of which 66 for COVID-19 vaccines have been selected (Figure 1).
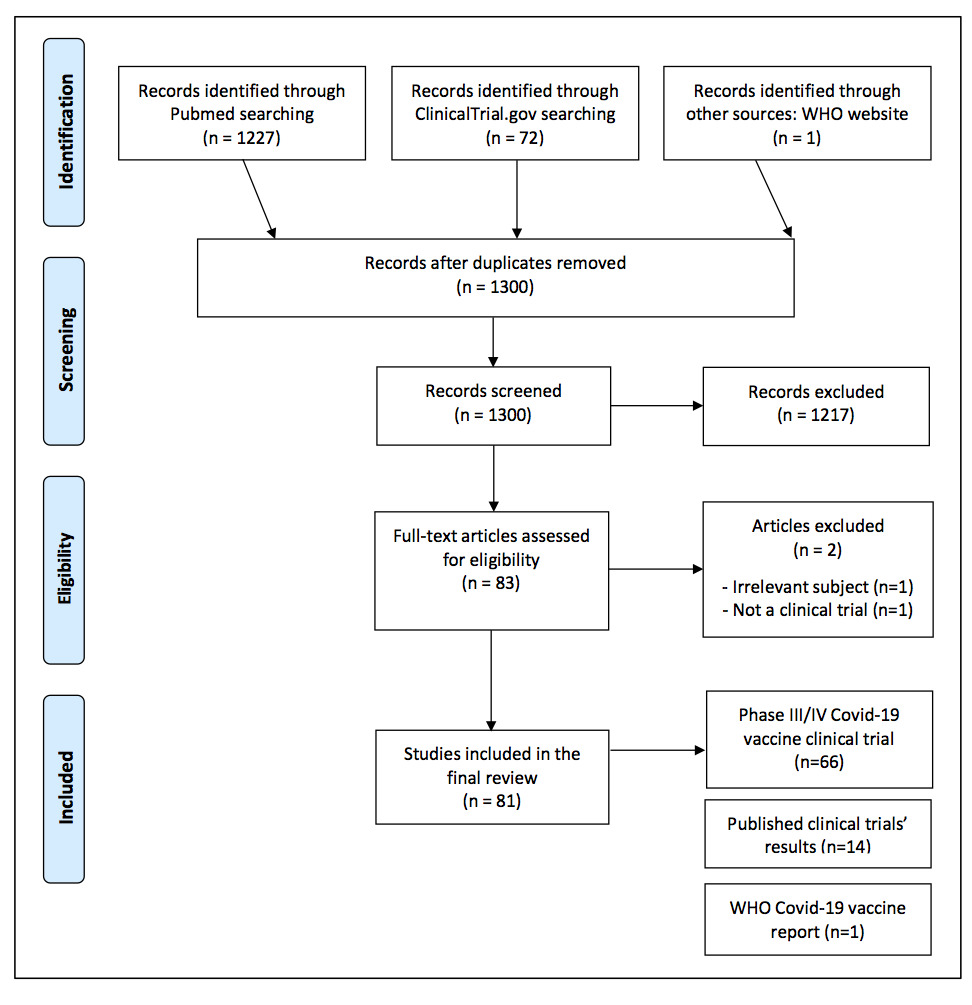
Figure 1:PRISMA flow chart.
WHO COVID-19 vaccine report as of January 24, 2021
According to the WHO COVID-19 vaccine report of January 22, 2021, there were 237 vaccines. More specifically, 64 vaccines were in clinical evaluation (16 in phase III, 6 in phase II / III, 5 in phase II, 19 in phase I / II and 18 in phase I) and 173 vaccines were in preclinical evaluation.3
Phase III and IV clinical trials
New COVID-19 Vaccines
Vaccine development, determined by clinical trials, is divided into three phases between preclinical exploratory work and vaccine approval (Box 1).4
Most of the candidate COVID-19 vaccines are based on the S antigen in the form of inactivated vaccines, subunit vaccines, viral vector vaccines, and DNA or mRNA 3 nucleic acid vaccines.5
In this review, the focus will be on clinical trials for vaccines in phase III and above.
For the new candidates COVID-19 vaccines, there were 37 clinical trials on Phase III, of which one clinical trial was simultaneously in phase I/II/III and eight in phase II/III. Eleven (30%) clinical trials concerned inactivated vaccines and 30 (81%) clinical trials used two doses. Six clinical trials were active and not recruiting while 23 were still recruiting participants. The number of participants to be recruited varied from 100 to 60,000 (Table 1).
Table 1:Candidate vaccines against COVID-19 in phase III as of January 24, 2021
|
COVID-19 vaccine developer / Manufacturer
|
Type of vaccine
|
Number of doses
|
Timing of doses
|
Route of administration
|
Country
|
Recruitment status
|
Estimated number of participants
|
Description of the clinical trial
|
|
BioNTech SE/Pfizer 6 |
RNA (3 LNP-mRNA) |
2 |
Day 0 and Day 21 |
IM |
Argentina, Brazil, Germany, South Africa, Turkey, United States |
Active, not Recruiting |
43 998 |
Phase I/II/III, randomized, triple-blind (participant, care provider, investigator), placebo-controlled clinical trial with participants randomly assigned to placebo and experimental vaccine cohorts (BNT162b1 and BNT162b2) in healthy individuals aged 12 years and over
The study consists of 2 parts:
• Phase I: to identify the preferred candidate vaccine (s) and dose levels
• Phase II/III: an enlarged cohort and an efficiency part
The candidate vaccine selected for Phase II/III evaluation was BNT162b2 in medium dose
ClinicalTrials.gov Identifier : NCT04368728
Estimated Study Completion Date : January 31, 2023
|
|
Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation 7 |
Combined viral vector (Adenovirus (rAd26-S + rAd5-S)) |
2 |
Day 0 and Day 21 |
IM |
Russian Federation |
Active, not recruiting |
33 758 |
Multicenter, randomized, double-blind, placebo-controlled clinical trial in healthy adults aged 18 to 111 years. After screening, they will be randomized (3:1) into two groups, a reference group of 10,000 volunteers receiving placebo and a study group of 30,000 volunteers receiving the Gam-COVID-Vac combined vector vaccine. The trial subjects will be randomized into five age strata: 18-30, 31-40, 41-50, 51-60, and 60+ years.
ClinicalTrials.gov Identifier : NCT04530396
Estimated Study Completion Date : May 1, 2021
|
|
ModernaTX/NIAD 8 |
RNA (Encapsulated LNP-mRNA) |
2 |
Day 0 and Day 28 |
IM |
United States |
Active, not recruiting |
30 000 |
Quadruple-blind (participant, caregiver, investigator, outcome assessor), placebo-controlled randomized clinical trial with participants randomly assigned to placebo and experimental vaccine cohorts (mRNA-1273) in healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04470427
Estimated Study Completion Date : October 27, 2022
|
|
Laboratory Elea Phoenix S.A 9 |
Inactivated |
2 |
Day 0 and Day 21 |
IM |
Argentina |
Active not recruiting |
3000 |
Double-blind, placebo-controlled clinical trial with randomly (1:1) participants assigned to placebo and experimental (Vero cell) vaccine cohorts in healthy adults aged 18 to 85 years.
ClinicalTrials.gov Identifier : NCT04560881
Estimated Study Completion Date : December 1, 2021
|
|
NPO Petrovax 10 |
Adenovirus type 5 Vector |
1 |
Day 0 |
IM |
Russian Federation |
Active, not recruiting |
500 |
Randomized, multicenter, quadruple-blind (participant, care provider, investigator, outcome assessor), placebo-controlled clinical trial with randomized (3:1) participants assigned in placebo (n=125) and experimental vaccine (n=375) cohorts in adults in good health aged 18 to 85 years.
ClinicalTrials.gov Identifier : NCT04540419
Estimated Study Completion Date : July 31, 2021
|
|
Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation 11 |
Combined viral vector (Adenovirus (rAd26-S + rAd5-S)) |
2 |
Day 0 and Day 21 |
IM |
Belarus |
Active, not Recruiting |
100 |
Double-blind, placebo-controlled clinical trial with participants randomly assigned (3:1) to placebo (n=25) and experimental (GAM-COVID-Vac; n=75) vaccine cohorts in healthy adults aged from 18 to 60 years old.
ClinicalTrials.gov Identifier : NCT04564716
Estimated Study Completion Date : April 10, 2021
|
|
Janssen Vaccines & Prevention B.V 12 |
Non-replicating viral vector |
1 |
Day 0 |
IM |
Argentina, Brazil, Chile, Colombia, Mexico, Peru, South Africa, United States |
Recruiting |
60 000 |
A randomized, quadruple-blind (participant, caregiver, investigator, outcome assessor), placebo-controlled clinical trial with participants randomly assigned to placebo and experimental vaccine (Ad26.COV2.S) cohorts in healthy adults aged 18 and older.
ClinicalTrials.gov Identifier: NCT04505722
Estimated Study Completion Date: March 10, 2023
|
|
China National Biotec Group Company Limited 13 |
Inactivated |
2 |
Day 0 and Day 21 |
IM |
Bahrain, Egypt, Jordan, United Arab Emirates, |
Recruiting |
45 000 |
Double-blind, placebo-controlled clinical trial with participants randomly assigned (1:1:1) to one cohort of placebo vaccine and two cohorts of experimental vaccine (Vero Cell) in healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier: NCT04510207
Estimated Study Completion Date : September 16, 2021
|
|
CanSino Biologics Inc/ Beijing Institute of Biotechnology 14 |
Non-replicating viral vector |
1 |
Day 0 |
IM |
Argentina, Chile, Mexico, Pakistan, Russian Federation |
Recruiting |
40 000 |
Double-blind, placebo-controlled clinical trial with randomly (1:1) participants assigned to placebo and experimental vaccine (Adenovirus Vector Type 5) cohorts in healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04526990
Estimated Study Completion Date: January 30, 2022
|
|
CureVac AG 15 |
mRNA |
2 |
Day 0 and Day 28 |
IM |
Belgium, Germany, Netherlands |
Recruiting |
36 500 |
A phase IIb/III, multicenter, randomized, double-blind, placebo-controlled clinical trial with participants randomized to placebo and experimental vaccine (CvnCoV)) cohorts in healthy adults aged 18 years and older..
ClinicalTrials.gov Identifier : NCT04652102
Estimated Study Completion Date : March 4, 2023
|
|
Chinese Academy of Medical Sciences 16 |
Inactivated |
2 |
Day 0 and Day 14 |
IM |
Brazil, Malaysia |
Recruiting |
34 020 |
Randomized, double-blind, placebo-controlled clinical trial with participants randomized (1:1) to placebo and experimental vaccine (Vero Cell) cohorts in healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04659239
Estimated Study Completion Date : March 2022
|
|
AstraZeneca 17 |
Non-replicating viral vector |
2 |
Day 0 and Day 28 |
IM |
United States, Argentina, Chile, Colombia, Peru, |
Recruiting |
30 000 |
Multicenter, quadruple-blind (participant, caregiver, investigator, outcome assessor) placebo-controlled clinical trial with randomized (2:1) participants to placebo and experimental (AZD1222) vaccine cohorts in healthy adults aged 18 years old and older.
ClinicalTrials.gov Identifier : NCT04516746
Estimated Study Completion Date : October 5, 2022
|
|
Medicago 18 |
Recombinant |
2 |
Day 0 and Day 21 |
IM |
United States, Canada |
Recruiting |
30 000 |
A phase II/III, randomized, double-blind, placebo-controlled clinical trial with participants randomized to placebo and experimental vaccine cohorts in healthy adults aged 18 years and older..
ClinicalTrials.gov Identifier : NCT04636697
Estimated Study Completion Date : April 30, 2022
|
|
Novavax 19 |
Recombinant Spike Protein Nanoparticle Vaccine (SARS CoV 2 rS) with Matrix-M1 TM Adjuvant |
2 |
Day 0 and Day 21 |
IM |
United States, Mexico, Puerto Rico |
Recruiting |
30 000 |
Quadruple-blind (participant, care provider, investigator, outcome assessor), placebo-controlled randomized clinical trial with participants randomly assigned to placebo and experimental vaccine (NVX-CoV2373) cohorts in healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04611802
Estimated Study Completion Date : December, 2022
|
|
Janssen Vaccines & Prevention B.V 20 |
Non-replicating viral vector |
2 |
Day 0 and Day 56 |
IM |
United States, Belgium, Colombia, France, Germany, Philippines, South Africa, Spain, United Kingdom |
Recruiting |
30 000 |
Double-blind, placebo-controlled randomized clinical trial with participants randomly assigned to placebo and experimental vaccine (Ad26.COV2.S) cohorts in healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04614948
Estimated Study Completion Date : May 11, 2023
|
|
Anhui Zhifei Longcom Biologic Pharmacy Co.,Ltd
21
|
Recombinant |
3 |
Day 0, Day 30 and Day 60 |
IM |
China |
Recruiting |
29 000 |
Quadruple-blind (participant, care provider, investigator, outcome assessor), placebo-controlled randomized (1:1) clinical trial with participants randomly assigned to placebo and experimental vaccine (CHO cell) cohorts in healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04646590
Estimated Study Completion Date : April, 2022
|
|
Baharat Biotech international Limited 22 |
Whole-virion inactivated |
2 |
- |
IM |
India |
Recruiting |
25 800 |
Double-blind, placebo-controlled randomized clinical trial with participants randomly (1:1) assigned to placebo and experimental vaccine (BBV152) cohorts in healthy adults aged 18 to 99 years.
ClinicalTrials.gov Identifier : NCT04641481
Estimated Study Completion Date : March 1, 2022
|
|
Novavax 23 |
Recombinant Spike Protein Nanoparticle Vaccine (SARS CoV 2 rS) with Matrix-M1 TM Adjuvant |
2 |
Day 0 and Day 21 |
IM |
United Kingdom |
Recruiting |
15 000 |
Quadruple-blind (participant, care provider, investigator, outcome assessor), placebo-controlled randomized clinical trial with participants randomly assigned to placebo and experimental vaccine (NVX-CoV2373) cohorts in healthy adults aged 18 to 84 years.
ClinicalTrials.gov Identifier : NCT04583995
Estimated Study Completion Date : January, 2022
|
|
Health Institutes of Turkey 24 |
Inactivated |
2 |
Day 0 and Day 14 |
IM |
Turkey |
Recruiting |
13 000 |
Double-blind, placebo-controlled clinical trial with randomized participants assigned to placebo and experimental vaccine cohorts (Vero-Cell) in healthy adults aged 18 to 59 years old.
ClinicalTrials.gov Identifier : NCT04582344
Estimated Study Completion Date : April 15, 2021
|
|
University of Oxford 25 |
Non-replicating viral vector |
1 or 2 |
If 2 doses, 4-6 weeks apart |
IM |
United Kingdom |
Recruiting |
12 390 |
Phase II /III, single-blind, controlled clinical trial, having 11 study groups with participants aged 18 years and older. Groups will receive the experimental vaccine (ChAdOx1 nCoV-19) and others the meningococcal vaccine (MenACWY).
ClinicalTrials.gov Identifier : NCT04400838
Estimated Study Completion Date : September 2021
|
|
Institute Butantan /Sinovac Life Sciences CO.,Ltd 26 |
Inactivated |
2 |
Day 0 and Day 14 |
IM |
Brazil |
Recruiting |
13 060 |
Double-blind, placebo-controlled clinical trial with randomized (1:1) participants assigned to placebo and experimental vaccine cohorts in healthy adults aged 18 years and older. For safety and immunogenicity, participants are categorized in 2 age groups: adults (18-59 years) and elderly (60 years and older).
ClinicalTrials.gov Identifier : NCT04456595
Estimated Study Completion Date : September 2021
|
|
University of Oxford 27 |
Non-replicating viral vector |
1 or 2 |
Day 0 or Day 0 and Day 28-90 |
IM |
Brazil |
Recruiting |
10 300 |
Single-blind, controlled clinical trial, with 2 study groups of healthcare professionals and adults with high potential for exposure to SARS-CoV-2, aged 18 years and older. One group will receive the experimental vaccine (ChAdOx1 nCoV-19) and the other will receive the meningococcal vaccine (MenACWY).
ClinicalTrials.gov Identifier : NCT04536051
Estimated Study Completion Date : September 2021
|
|
University Peruana Cayetano Heredia 28 |
Inactivated |
2 |
Day 0 and Day 14 |
IM |
Peru |
Recruiting |
6000 |
Multicenter, double-blind, placebo-controlled clinical trial with randomized participants assigned to placebo and experimental vaccines cohorts in healthy adults aged 18 to 60 years old.
ClinicalTrials.gov Identifier : NCT04612972
Estimated Study Completion Date : September 2021
|
|
Research Institute for Biological Safety Problems 29 |
Inactivated |
2 |
Day 0 and Day 21 |
IM |
Kazakhstan |
Enrolling by invitation |
3000 |
Multicenter, double-blind, placebo-controlled clinical trial with randomized participants assigned to placebo (n=600) and experimental vaccine cohorts QazCovid-in® (n=2400) in healthy adults aged 18 years old and older.
ClinicalTrials.gov Identifier : NCT04691908
Estimated Study Completion Date : July 31, 2021
|
|
ModernaTX, Inc 30 |
mRNA |
2 |
Day 0 and Day 28 |
IM |
United States |
Recruiting |
3000 |
A phase II/III, quadruple-blind, placebo-controlled clinical trial with randomized participants assigned to placebo and experimental vaccine (mRNA-1273) cohorts in healthy adolescents aged 12 to 17 years old
ClinicalTrials.gov Identifier : NCT04649151
Estimated Study Completion Date : June 30, 2022
|
|
CureVac AG 31 |
mRNA |
2 |
Day 0 and Day 28 |
IM |
Germany |
Recruiting |
2520 |
Double-blind, placebo-controlled clinical trial with randomized participants (1:1) assigned to placebo and experimental vaccine (CvnCoV) cohorts in healthy adults aged 18 years old and older.
ClinicalTrials.gov Identifier : NCT04674189
Estimated Study Completion Date : April 30, 2022
|
|
Pontifica Unversidad Catolica de Chile 32 |
Inactivated |
2 |
Day 0 and Day 14 |
IM |
Chile |
Recruiting |
2300 |
Multicenter, double-blind, placebo-controlled clinical trial with randomized participants (1:1) assigned to placebo and experimental vaccine cohorts in healthy adults aged 18 years old and older.
ClinicalTrials.gov Identifier : NCT04651790
Estimated Study Completion Date : March 2022
|
|
PT Bio Farma 33 |
Inactivated |
2 |
Day 0 and Day 14 |
IM |
Indonesia |
Recruiting |
1620 |
Randomized, double-blind, placebo-controlled clinical trial with participants randomized (1:1) to placebo and experimental vaccine cohorts in healthy adults aged 18 to 59 years.
ClinicalTrials.gov Identifier : NCT04508075
Estimated Study Completion Date : September 2021
|
|
Sinovac Biotech Co., Ltd 34 |
Inactivated |
2 |
Day 0 and Day 14 |
IM |
China |
Recruiting |
1040 |
Randomized, double-blind, placebo-controlled clinical trial to evaluate the non-inferiority of the commercial scale Inactivated SARS-CoV-2 vaccine against that of the pilot scale among healthy adults aged 26-45 years, and the open-labelled, bridging non-inferiority of the vaccine induced immunogenicity in healthy elderly (aged 60 years and older) against that in healthy adults (18-59 years old).
ClinicalTrials.gov Identifier : NCT04617483
Estimated Study Completion Date : May 31, 2021
|
|
AnGes, INC 35 |
DNA |
2 |
Day 0 and Day 14 or Day 0 and Day 28 |
IM |
Japan |
Recruiting |
500 |
A phase II/III, randomized, double-blind, placebo-controlled clinical to assess safety, immunogenicity, and efficacy of twice dosing of IM AG0302-Covid-19 (2mg) in healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04655625
Estimated Study Completion Date : March 31, 2022
|
|
Clover Biopharmaceuticals AUS Pty Ltd 36 |
Adjuvanted recombinant Subunit vaccine |
2 |
Day 0 and Day 21 |
IM |
Belgium, Brazil, Colombia, Dominican Republic, Germany, Nepal, Panama, Philippines, Poland, South Africa |
Not yet recruiting |
34 000 |
A Phase II/III, double-blind, placebo-controlled clinical trial with randomized participants to placebo and experimental vaccine (AS03-adjuvanted SCB-2019) cohorts in healthy adults aged 18 years old and older.
ClinicalTrials.gov Identifier : NCT04672395
Estimated Study Completion Date : March, 2022
|
|
COVAXX 37 |
Protein subunit |
2 |
Day 0 and Day 28 |
IM |
- |
Not yet Recruiting |
7320 |
A phase II/III, multicenter, double-blind, placebo-controlled, dose-response study to evaluate the safety, immunogenicity and efficacity of UB-612 in age groups, adults aged 18 to 59 years and elderly aged more than 60 years with or without comorbidities.
ClinicalTrials.gov Identifier : NCT04683224
Estimated Study Completion Date : March 22, 2023
|
|
Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation 38 |
Combined viral vector (Adenovirus (rAd26-S + rAd5-S)) |
2 |
Day 0 and Day 21 |
IM |
Venezuela |
Not yet Recruiting |
2000 |
Double-blind, placebo-controlled clinical trial with participants randomly assigned (3:1) to placebo (n=500) and experimental (GAM-COVID-Vac; n=1500) vaccine cohorts in healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04642339
Estimated Study Completion Date : December, 2021
|
|
Dr Reddy’s Laboratories Limited / Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation 39 |
Combined viral vector |
2 |
Day 0 and Day 21 |
IM |
India |
Not yet Recruiting |
1600 |
A phase II/III, double-blind, placebo-controlled clinical trial with participants randomly assigned (3:1) to experimental (GAM-COVID-Vac) vaccine and placebo cohorts in healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04640233
Estimated Study Completion Date : September, 2021
|
|
Pfizer/ BioNTech SE 40 |
RNA |
2 |
Day 0 and Day 21 |
IM |
- |
Not yet Recruiting |
1280 |
Double-blind, placebo-controlled clinical trial with participants randomly assigned to placebo and experimental vaccine (BNT162B2) cohorts in healthy adults aged 18 to 55 years.
ClinicalTrials.gov Identifier : NCT04713553
Estimated Study Completion Date : April 9, 2021
|
|
Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation 41 |
Combined viral vector |
1 |
Day 0 |
IM |
United Arab Emirates |
Not yet Recruiting |
1000 |
Double-blind, placebo-controlled clinical trial with participants randomly assigned (3:1) to placebo (n=250) and experimental (GAM-COVID-Vac; n=750) vaccine cohorts in healthy adults aged 18 years and older. The trial subjects will be randomized into five age strata: 18-30, 31-40, 41- 50, 51-60, and 60+ years
ClinicalTrials.gov Identifier : NCT04656613
Estimated Study Completion Date : December, 2021
|
|
AstraZeneca 42 |
Non-replicating viral vector |
2 |
Day 0 and Day 28 |
IM |
Russian Federation |
Suspended |
100 |
Multicenter, unblinded, uncontrolled clinical trial in which all participants receive the experimental vaccine (AZD1222). Included are healthy adults aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04540393
Estimated Study Completion Date : Mach 12, 2021
|
Other vaccines to fight COVID-19
Measles vaccine
With the hypothesis that the measles vaccine could reduce the incidence of COVID-19, as of January 24, 2021, there were three clinical trials, in phase III, using this vaccine in the fight against infection with SARS-CoV-2:
-
An international clinical trial (NCT04333732), randomized, double-blind, placebo-controlled, in healthcare workers at risk of contracting COVID-19 (N = 30 000) and aged 18 years and older. These are randomly assigned to cohorts of placebo vaccine and MMR or MR vaccine. The estimated end of study date is August 2021.43
-
A randomized, single-blinded, placebo-controlled clinical trial in New Orleans (NCT04475081), recruiting healthy healthcare workers (N=60) aged 18 to 70 years. They are randomly assigned to cohorts of placebo vaccine and MMR vaccine. The estimated end of study date is December 1st, 2021.44
-
A randomized clinical trial in Egypt (NCT04357028), single-blind, placebo-controlled, recruiting healthy healthcare workers (N = 200) and aged between 18 and 50 years. Participants are randomly assigned to cohorts of placebo vaccine and MMR vaccine. The estimated end of study date was November 1st, 2020, but the clinical trial was suspended due to failure of subject recruitment.45
Polio vaccine
Both polio and coronavirus are positive strand RNA viruses, it is likely that they can induce common innate immune mechanisms. There were three clinical trials:
-
A randomized clinical trial in Guinea-Bissau (NCT04445428), double-blind and in phase IV, evaluating the effect of the administration of the oral polio vaccine (OPV) compared to the absence of vaccine in 3400 people, aged over 50 years and recruited by invitation. The objective of the trial is to test the hypothesis that OPV reduces the combined risk of admission or death from morbidity by at least 28% over the next six months. The estimated end of this study date is December 2021.46
-
A Phase IV clinical trial in United States (NCT04639375), evaluating whether an immune response to SARS-CoV 2 RdRp is induced in adults after receiving a booster inoculation of IPV. The number of participants is estimated at 25 healthy volunteers aged between 18 and 80 years old. All participants will receive one IPV by injection. The estimated end of this study date is January 15, 2021.47
-
A randomized, multi-center, phase III, clinical trial in the United States and New Zealand (NCT04540185), evaluating the safety and efficacy of OPV with and without NA-831 versus to placebo. The number of participants is estimated at 3600, aged over 18 years, in good health and recruited by invitation.48
BCG vaccine
Hypotheses have been made that the tuberculosis vaccine (BCG) may induce partial protection against the severity of infection with SARS-CoV-2. In this context, as of January 24, 2021, there were 23 clinical trials using the BCG vaccine, including six in phase IV.49–54 Four (17%) clinical trials were active and not recruiting,49,55–57 while the Colombian clinical trial was withdrawn due to the lack of sponsorship.58 The number of recruits ranged from 59 to 10 078 (Table 2).
Published clinical trials’ results
As of January 24, 2021, there was 13 clinical trials’ published results in different phases, of which six were in phase I/II59–64 and four had a number of participants less than 100.60,64–66 Only two clinical trials had a non-randomized trial.60,67 All studies concerned healthy adults aged 18 years and over. In general, local and systemic reactions if present, were described as mild to moderate and consisted in injection site pain, fever, myalgia, headache and fatigue (Table 3).
At this time, there is no determination as to whether a vaccine candidate will be universal or indicated for specific populations, or how many doses will be needed, or the likely presentations.68
Table 2:Phase III and IV clinical trials using the tuberculosis vaccine for the fight against COVID-19 as of January 24, 2021
|
COVID-19 vaccine developer / Manufacturer
|
Clinical Trial phase
|
Number of Doses
|
Timing of doses
|
Route of administration
|
Country
|
Recruitment Status
|
Estimated number of participants
|
Description of the clinical trial
|
|
Radboud University 49 |
Phase IV |
1 |
Day 0 |
ID |
Netherlands |
Active ,not recruiting |
2014 |
A randomized, multicenter, single-blind, placebo-controlled clinical trial with participants randomly assigned (1:1) to one BCG vaccine cohort and another placebo cohort in healthy subjects aged 60 years and older.
ClinicalTrials.gov Identifier : NCT04417335
Estimated Study Completion Date : May 2021
|
|
UMC Utrecht 50 |
Phase IV |
1 |
Day 0 |
ID |
Netherlands |
Recruiting |
5200 |
Randomized, multicenter, quadruple-blind (participant, care provider, investigator, outcome assessor) placebo-controlled clinical trial with participants randomized (1:1) to one BCG vaccine cohort and another placebo cohort in subjects aged 60 years and older, with a medical history.
ClinicalTrials.gov Identifier : NCT04537663
Estimated Study Completion Date : April 2021
|
|
Texas A&M University 51 |
Phase IV |
1 |
Day 0 |
ID |
United States |
Recruiting |
1800 |
Randomized, multicenter, double-blind, placebo-controlled clinical trial with participants randomly assigned (1:1) to a BCG vaccine cohort and an another placebo cohort in high risk healthy healthcare workers with direct COVID-19 infected patients contacts and aged between 18 and 75 years.
ClinicalTrials.gov Identifier : NCT04348370
Estimated Study Completion Date: November 2021
|
|
University of Southern Denmark 52 |
Phase IV |
1 |
Day 0 |
ID |
Cape Verde, Guinea-Bissau, Mozambique |
Recruiting |
1050 |
Randomized, multicenter, double-blind, placebo-controlled clinical trial with participants randomly (1:1) assigned to a BCG vaccine cohort and another placebo cohort in healthy healthcare workers aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04641858
Estimated Study Completion Date : March 2022
|
|
University of Campinas, Brazil 53 |
Phase IV |
1 |
Day 0 |
ID |
Brazil |
Recruiting |
1000 |
Randomized, multicenter, double-blind, placebo-controlled clinical trial with participants randomly assigned (1:1) to one BCG vaccine cohort and another placebo cohort in COVID-19 positive subjects aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04369794
Estimated Study Completion Date : August 2023
|
|
Hellenic Institute for the Study of Sepsis 54/td>
|
Phase IV |
1 |
Day 0 |
ID |
Greece |
Recruiting |
900 |
Quadruple-blind randomized clinical trial (participant, care provider, investigator, outcome assessor) placebo-controlled with healthy participants aged 50 years and older and randomly assigned (1:1) to one BCG vaccine cohort and another placebo cohort.
ClinicalTrials.gov Identifier: NCT04414267
Estimated Study Completion Date : May 25, 2021
|
|
UMC Utrecht/ Radboud University 55 |
Phase III |
1 |
Day 0 |
ID |
Netherlands |
Active , not recruiting |
1500 |
Placebo-controlled, randomized, quadruple-blind (participant, provider, investigator, outcome assessor) clinical trial with participants randomly (1:1) assigned to a BCG vaccine cohort and a placebo cohort among healthy healthcare workers in hospitals, aged 18 years and older, and caring for COVID-19 positive patients.
ClinicalTrials.gov Identifier : NCT04328441
Estimated Study Completion Date : April 30, 2021
|
|
Hospital University Dr. Jose E. Gonzalez 56 |
Phase III |
1 |
Day 0 |
ID |
Mexico |
Active ,not recruiting |
908 |
Multicenter, randomized, triple-blind, placebo-controlled clinical trial with participants randomly assigned (1:1) to one BCG vaccine cohort and another placebo cohort in healthcare workers aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04461379
Estimated Study Completion Date : January 1, 2021
|
|
Vakzine Projekt Management GmbH 57 |
Phase III |
1 |
Day 0 |
ID |
Germany |
Active not recruiting |
59 |
Randomized, triple-blind, placebo-controlled clinical trial with participants randomly assigned (1:1) to one BCG vaccine (VPM1002) cohort and an another placebo cohort in hospital workers with high exposure to SARS-CoV-2, healthy and aged 18 years old and older.
ClinicalTrials.gov Identifier : NCT04387409
Estimated Study Completion Date : May 1, 2021
|
|
Murdoch Children’s Research Institute 69 |
Phase III |
1 |
Day 0 |
ID |
Australia, Netherland, Spain, United Kingdom |
Recruiting |
10 078 |
Randomized, multicenter, double-blind, placebo-controlled clinical trial with participants randomly assigned to a BCG vaccine cohort and another placebo cohort in healthy healthcare workers aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04327206
Estimated Study Completion Date : March 30, 2022
|
|
University Health Network, Toronto 70 |
Phase III |
1 |
Day 0 |
ID |
Canada |
Recruiting |
3626 |
Randomized, double-blind, placebo-controlled clinical trial with participants randomly assigned to a BCG vaccine (VPM1002) cohort and another placebo cohort among frontline employees (municipal or provincial police, emergency medical services, fire departments, public transport service, health service, food manufacturing facility) aged 18 years and over.
ClinicalTrials.gov Identifier : NCT04439045
Estimated Study Completion Date : July 1, 2021
|
|
Tuberculosis Research Center, India 71 |
Phase III |
1 |
Day 0 |
ID |
India |
Recruiting |
2175 |
Non-randomized, unblinded clinical trial with healthy participants aged between 60 and 80 years, assigned (2:1) to one BCG vaccine cohort and one without vaccination.
ClinicalTrials.gov Identifier : NCT04475302
Estimated Study Completion Date : May 2021
|
|
Vakzine Projekt Management GmbH 72 |
Phase III |
1 |
Day 0 |
ID |
Germany |
Recruiting |
2038 |
Randomized, multicenter, triple-blind, placebo-controlled clinical trial with participants randomly assigned (1:1) to one BCG vaccine cohort (VPM1002) and another placebo cohort in healthy adults aged 60 years and older.
ClinicalTrials.gov Identifier : NCT04435379
Estimated Study Completion Date : September 30, 2021
|
|
Bandim Health Project 73 |
Phase III |
1 |
Day 0 |
ID |
Denmark |
Recruiting |
1900 |
Randomized, double-blind, placebo-controlled clinical trial with participants randomly assigned (1:1) to one BCG vaccine cohort and an another placebo cohort in healthy adults aged 65 years and older.
ClinicalTrials.gov Identifier : NCT04542330
Estimated Study Completion Date : March 2022
|
|
Bandim Health Project 74 |
Phase III |
1 |
Day 0 |
ID |
Denmark |
Recruiting |
1500 |
Randomized, double-blind, placebo-controlled clinical trial with participants randomly assigned (1:1) to a BCG vaccine cohort and a placebo cohort in healthy healthcare workers with direct contact with patients with COVID-19 and aged 18 years to 100 years.
ClinicalTrials.gov Identifier : NCT04373291
Estimated Study Completion Date : August, 2021
|
|
Assistance Publique- Hôpitaux de Paris 75 |
Phase III |
1 |
Day 0 |
ID |
France |
Recruiting |
1120 |
Randomized, single-blind, placebo-controlled clinical trial with participants randomly assigned (1:1) to one BCG vaccine cohort and another placebo cohort in healthy healthcare workers treating patients with COVID-19 and aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04384549
Estimated Study Completion Date : February 20, 2021
|
|
Hanna Czajka, University of Rzeszow 76 |
Phase III |
1 |
Day 0 |
ID |
Poland |
Recruiting |
1000 |
Multicenter, placebo-controlled, randomized, Partially double blinded clinical trial with participants randomly assigned (1:1) to one BCG vaccine cohort and another placebo cohort among healthy health care workers aged 25 years and older.
ClinicalTrials.gov Identifier : NCT04648800
Estimated Study Completion Date : April , 2021
|
|
Henry M. Jackson Foundation for the advancement of Military Medicine 77 |
Phase III |
1 |
Day 0 |
ID |
United States |
Recruiting |
550 |
Multicenter, placebo-controlled, randomized, triple-blind (participant, care provider, investigator) clinical trial with participants randomly assigned (1:1) to one BCG vaccine cohort (Tice® BCG) and another placebo cohort among healthy healthcare workers who are likely to care for Covid-19 patients and aged 18 to 64 years old.
ClinicalTrials.gov Identifier : NCT04632537
Estimated Study Completion Date : April , 2023
|
|
TASK Applied Science 78 |
Phase III |
1 |
Day 0 |
ID |
South Africa |
Recruiting |
500 |
Placebo-controlled, randomized, quadruple-blind (participant, provider, investigator, outcome assessor) clinical trial with participants randomly assigned (1:1) to one BCG vaccine cohort and another placebo cohort among healthy healthcare workers aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04379336
Estimated Study Completion Date :April 28, 2021
|
|
Fundació Institute Germans Trias i Pujol 79 |
Phase III |
2 |
Day 0 and Day 14 |
ID |
Spain |
Not yet recruiting |
315 |
Quadruple-blind randomized clinical trial (participant, provider, investigator, outcome assessor) placebo-controlled trial with participants randomly assigned to cohorts of placebo vaccine and RUTI® vaccine in healthy healthcare workers aged 18 years old and older.
ClinicalTrials.gov Identifier : NCT04453488
Estimated Study Completion Date : December 2020
|
|
Harvard Medical School 80 |
Phase III |
1 |
Day 0 |
ID |
- |
Not yet recruiting |
2100 |
Randomized, triple-blind, placebo-controlled clinical trial with participants randomly assigned (1:1) to BCG vaccine and placebo cohorts in healthy subjects aged 70 years and older and resident in a long-term care facilities.
ClinicalTrials.gov Identifier : NCT04534803
Estimated Study Completion Date : November 30, 2021
|
|
Ain Shams University 81 |
Phase III |
1 |
Day 0 |
ID |
Egypt |
Not yet recruiting |
900 |
Multi-center, randomized, single-blind clinical trial with participants randomly assigned (2: 1) to a BCG vaccine cohort (n=600) and a placebo cohort (n=300) in healthy healthcare workers working in the isolation hospitals for COVID-19 cases and aged 18 years and older.
ClinicalTrials.gov Identifier : NCT04350931
Estimated Study Completion Date : December 1, 2020
|
|
University of Antioquia 58 |
Phase III |
1 |
Day 0 |
ID |
Colombia |
Withdrawn
(Principal Investigator did not obtain sponsorship to carry it out)
|
- |
Randomized, multicenter, double-blind, placebo-controlled clinical trial with participants randomized (1:1) to BCG vaccine and placebo cohorts in healthy adults aged 18 to 65 years.
ClinicalTrials.gov Identifier : NCT04362124
Estimated Study Completion Date: November 2021
|
Table 3:COVID-19 vaccine clinical trials published results, as of January 24, 2021
|
COVID-19 vaccine developer / Manufacturer
|
Identifier
|
Phase
|
Brief description
|
Vaccine
|
Country
|
Number of participants
|
Published Results
|
Status of the vaccine
|
References
|
|
CanSino Biologics Inc.
|
NCT04313127
|
I |
A non-randomized clinical trial, in healthy adults (18-60 years) |
Recombinant adenovirus type 5 vector |
Wuhan, China |
108 |
The vaccine was tolerable, immunogenic 28 days after vaccination and that rapid specific T lymphocytes were noted from day 14 post vaccination.
Most adverse reactions were mild or moderate in severity. The most common were pain In injection side (54%), fever (46%), fatigue (44%), headache (39%) and muscle pain (17%).
|
Registered in phase III (NCT04526990)14 and recruiting participants |
Zhu et al 67 |
|
Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China
|
NCT04341389
|
II |
Randomized, placebo controlled clinical trial in healthy adults aged 18 years and older |
Recombinant adenovirus type 5 vector |
Wuhan, China |
508 |
The 5 × 1010 viral particle vaccine was safe and elicited significant immune responses in the majority of recipients after a single immunization |
Registered in phase III (NCT04526990)14 and recruiting participants |
Zhu et al 82 |
|
National Institute of Allergy and Infectious Diseases (NIAID)
|
NCT04283461
|
I |
An open-label dose-ranging of the safety and immunogenicity of COVID-19 vaccine in healthy adults aged 18 years and over |
mRNA-1273 |
United States |
45 (preliminary report)
40
|
Its preliminary report including 45 healthy adults aged 18 to 55 years, stated that the vaccine induced an immune response in all participants and that no trial-limiting safety concerns were identified.
Due to the high mortality and incidence of Covid-19 in older population, the trial was expanded to include 40 older adults aged 56 years and older. It was concluded that the mRNA-1273 had mainly mild to moderate adverse events in older adults and that the 100-μg dose induced higher binding- and neutralizing-antibody titers than the 25-μg dose.
|
Registered in phase III (NCT04470427) 8 and active not recruiting |
Jackson et al 65
Anderson et al 83
|
|
Beijing Institute of Biological Products Co., LTD
|
ChiCTR2000032459 |
I/II |
A randomized, double-blind, placebo-controlled clinical trial in healthy adults aged 18 years and older (18-80 years in phase I and 18-59 years in phase II) |
Inactivated vaccine (BBIBP-CorV) |
Henan Province, China |
1192 in phase I
2448 in phase II
|
it was reported that the vaccine was safe, well tolerated and that the two-dose immunization with 4 μg vaccine on days 0 and 21 or days 0 and 28 achieved higher neutralizing antibody titers than the single 8 μg dose or 4 μg dose on days 0 and 14. Adverse reactions were mild or moderate in severity. |
- |
Xia et al 59 |
|
Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation
|
NCT04436471
NCT04437875
|
I/II |
Two open-label, non-randomized clinical trial in healthy volunteers aged 18 to 60 years, aiming to assess the safety and immunogenicity of two formulations (frozen and lyophilized) of the vaccine |
Recombined viral vector vaccine (rAd26 and rAd5) |
Russia |
76 (38 in each study) |
It was reported that the vaccine was safe and induced strong humoral and cellular immune responses in participants.
The most common adverse events were pain at injection site (58%), hyperthermia (50%), headache (42%), asthenia (28%), and muscle and joint pain (24%). Most adverse events were mild and no serious adverse events were detected
|
The vaccine is in phase III with five clinical trials (NCT04530396, NCT04564716, NCT04642339, NCT04640233 and NCT04656613) 7,11,38,39,41
|
Logunov et al 60 |
|
University of Oxford
|
NCT04324606
|
I/II |
Randomized, single-blind, phase I/II clinical trial controlled by a meningococcal vaccine, in healthy adults aged 18 to 55 years. |
Non-replicating viral vector (ChAdOx1 nCoV-19) |
United-Kingdom |
1077 |
It was reported that the ChAdOx1 nCoV-19 vaccine had an acceptable safety profile and that a booster dose boosted antibody responses.
Local and systemic reactions were more common in the COVID-19 vaccine group including pain, feeling feverish, chills, muscle ache, headache, and malaise.
|
The vaccine is in phase II/III (NCT04400838) 25 and recruiting participants |
Folegatti et al 61 |
|
University of Oxford
|
NCT04400838 25 |
II/III |
A single-blind, randomized, controlled, in healthy adults aged 18 years and older enrolled at two UK clinical research facilities, in an age-escalation manner (18–55 years, 56–69 years, and 70 years and older immunogenicity subgroups) |
Non-replicating viral vector (ChAdOx1 nCoV-19) |
United-Kingdom |
560
(160 aged 18-55 years, 160 aged 56-69 years and 240 aged 70 years and older)
|
The preliminary stated that ChAdOx1 nCoV-19 appears to be better tolerated in older adults than younger adults. Local and systemic reactions (injection-site pain, feeling feverish, myalgias, headache) were less common for those aged 56 years and older. It was also reported that the vaccine had similar immunogenicity across all age groups after a booster dose. |
Recruiting participants |
Ramasamy et al 84 |
|
Novavax
|
NCT04368988
|
I/II |
Randomized, placebo-controlled clinical trial in healthy adults aged 18 to 59 years |
Recombinant spike protein nanoparticle vaccine with Matrix-M1 adjuvant (NVX-CoV2373) |
Australia |
131 |
The primary analysis at day 35 concluded that that the vaccine appeared safe, elicited immune responses that exceeded levels in Covid-19 convalescent serum and that the adjuvant induced CD4+ T-cell responses biased toward a Th1 phenotype. Reactogenicity was absent or mild in the majority of participants, more common with adjuvant |
The vaccine has two phase II clinical trials (NCT04611802 and NCT04583995) 19,23
|
Keech et al 62 |
|
BioNTch/Pfizer’s
|
NCT04368728
|
I |
Placebo-controlled, observer-blinded and dose escalation clinical trial in healthy adults aged 18 to 55 years and 65 to 85 years |
Two RNA vaccines (BNT162b1 and BNT162b2) |
United-States |
195 |
The trial supported the selection of BNT162b2 for advancement to a pivotal phase II/III safety and efficacy evaluation |
- |
Walsh et al 85 |
|
|
I/II |
Ongoing placebo-controlled, observer-blinded, dose-escalation in healthy adults aged 18 to 55 years |
RNA vaccines (BNT162b1) |
United-States |
45 |
It reported that the vaccine exhibited a tolerability and safety profile consistent with those previously observed for mRNA-based vaccine. Local reactions and systemic events were dose-dependent, generally mild to moderate, and transient. However, even if there was stated that there was a robust immunogenicity after vaccination with BNT162b1, the findings were not proof of vaccine efficacy |
- |
Mulligan et al 63 |
|
|
II/III |
Ongoing multinational, placebo-controlled, observer-blinded clinical trial in healthy adults aged 16 years and older |
RNA vaccines (BNT162b2) |
United-States, Argentina, Brazil, South Africa, Germany, Turkey |
43 548 |
The preliminary report stated that a two dose regimen of BNT162b2 was 95% (95% CI [90.3%-97.6%]) effective against Covid-19 in persons aged 16 years and older. Safety over two months was similar to that of other viral vaccines and was characterized, especially after the second dose, by mild to moderate local reactions (pain, erythema, swelling) and systemic reactions (fever, headache, myalgias) which resolved rapidly |
- |
Polack et al 86 |
|
Wuhan Institute of Biological Products co., LTD.
|
ChiCTR2000031809
|
I and II |
Ongoing randomized, double-blind, placebo-controlled in healthy adults aged 18 to 59 years |
Inactivated vaccine |
Henan Province, China |
96 (phase I)
224 (phase II)
|
The interim analysis demonstrated immunogenicity in patients as well as a low rate of adverse reactions. The most common adverse reaction was injection site pain, followed by fever. No serious adverse reactions were noted |
This vaccine is in phase III (NCT04510207) 13 and recruiting participants |
Xia et al 66 |
|
BioNTech SE
|
NCT04380701
|
I/II |
An ongoing placebo controlled, observer-blinded clinical trial in healthy adults aged between 18 and 55 years |
RNA vaccines (BNT162b1) |
Germany |
60 |
It was reported that the vaccine was safe, tolerable and had antibody response. Reactogenicity was dose-dependent and more pronounced after the boost dose. Side effects such as fever, chills, headache, muscle pain, joint pain, injection site pain, and tenderness, was mostly mild or moderate. The report concluded that BNT162b1 induced functional and proinflammatory CD4+ and CD8+ T cell responses in almost all participants, with TH1 polarization of the helper response |
- |
Sahin et al 64 |
DISCUSSION
Since the emergence of SARS-CoV-2, the scientific community has been committed to identifying a therapeutic approach as well as a vaccine that is both effective and safe to achieve the goal of reducing morbidity and mortality attributable to COVID-19.
This review is a recent synthesis of clinical trials and published protocols regarding COVID-19 vaccination.
In total, as of January 24, 2021, there were 37 phase III clinical trials involving new candidates for the COVID-19 vaccine. The number of subjects required to be included in these trials varied between 100 and 60 000. The criteria for non-inclusion and exclusion in these phase clinical trials III were mainly children and pregnant women: none of the protocols of these trials concerned pregnant women and almost all of them didn’t include persons aged less than 18 years, except for the multinational BioNTech SE/Pfizer’s clinical trial where they included healthy individuals aged 12 years and over for their phase II/III.6 and ModernaTX’s clinical trial including healthy adolescents aged 12 to 17 years.30 AstraZenaca’s clinical trial in Russia was suspended due the occurrence of suspected unexpected serious adverse reaction at University of Oxford sponsored study, and will continue to be on hold until the approval on the Russian Ministry of health.42
Under the hypothesis that certain other vaccines can strengthen innate immunity, thus making it possible to induce a decrease in infection by SARS CoV2, several other phase III and IV clinical trials have been launched such as BCG vaccine (n=23), measles vaccines (n=3) and polio vaccines (n=3). At the time of writing this review, there was no publication of the results of these clinical trials.
All published clinical trials’ results concerning the safety and immunogenicity of the vaccine were phases I/II and II/III with promising results concerning these two criteria. Having multiples vaccines candidates could be an advantage. In fact, in case one of them fails, there is still others under development. However, the majority of the published clinical trials’ results involved a small proportion of participants, in addition to the accelerated process of COVID-19 vaccines development, these could mask some side-effects. Moreover, this rush in the manufacturing process might lead to the production of a vaccine with limited effectiveness and therefore provide immunity, complete or incomplete, only to some vaccinated individuals.87 Furthermore, published clinical trials’ results involved only healthy individuals and few elderly. Even though the more vulnerable individuals, such as the elderly and those with co-morbidities would have priority for vaccines, the vaccine effectiveness and side effects are unknow in this group.87
Despite the progress made and the promising results of clinical trials of candidate vaccines, many obstacles and difficulties must be considered, in particular the logistical difficulties surrounding the mass production and the delivery of millions or even billions of doses to the world population, which will represent probably the biggest current challenge. Note that other constraints related to certain types of vaccines, such as mRNAs which are quite unstable at room temperature, requiring storage in freezers.88 Indeed almost all the new COVID-19 vaccines mentioned require cold storage with different requirements. The distribution of these vaccines then assumes a need for the cooperation of government and companies for cold storage and global transport. The Pfizer vaccine should be stored at a temperature of minus 70 °C ± 10 °C,89,90 the frozen Gam-COVID- vaccine (Sputnik V) requires a storage temperature of minus 18 °C, while its lyophilized formulation should be stored at a temperature of 2 to 8 °C.60 Furthermore, despite the rapid development and production of vaccines, their distribution to the most vulnerable and deprived populations and in the poorest countries remains the major issue requiring to help these groups and countries in order to benefit from the necessary and efficient logistics allowing to end this pandemic.
COVID-19 vaccine development continues at unprecedented speed, but it’s uncertain that there would be enough production in 2021. New waves of SARS-CoV-2 infections are likely to occur, therefore, we will have to continue preventive measures such as social distancing, mask wearing and hand hygiene.
Box 1:Vaccine development through phases of clinical trials
Phase I : These are the first experiments in humans on a small number of healthy volunteers and focusing on the safety and immunogenicity of the vaccine
Phase II : This phase covers a larger cohort and focuses more on the immunogenicity of the vaccine, allowing the immune response to be analyzed according to age, sex, ethnicity and other variables. Efficiency can also be assessed at this stage.
Parallel phase I/II and phase II/III studies can be performed, if sufficient data has been extracted from the previous phase, to achieve ethical acceleration.
Phase IV concerns post-marketing authorization trials.
Acknowledgements
We would like to thank WHO Tunisia Office for their financial support to the open access fees of this article.
Funding
None
Authorship contributions
MO performed the literature search and drafted the manuscript.
MS was responsible for the redaction and revision of the manuscript, and developed the methodology with MO.
AH and HL selected the clinical trials for new candidates COVID-19 vaccines and summarized it.
SDe and HBS were responsible for the other COVID-19 vaccine part, while SDh and CH were responsible for the published clinical trials’ results.
LB, SB and NBAB critically revised the manuscript.
All authors approved the final version of the manuscript.
Competing interest
The authors completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author), and declare no conflicts of interest.
Correspondence to
Molka Osman, National Observatory of New and Emerging Diseases, Tunisia, [email protected]