Intravaginal practices (IVPs) are behaviours undertaken by women for the promotion of hygiene and sexual health in sub-Saharan Africa, South-East Asia, and North and South America. A classification of different IVPs has been proposed by the World Health Organization (WHO) Gender, Sexuality and Vaginal Practices (GSVP) Study Group1,2 (Box 1). Use of IVPs can alter the vaginal micro-environment, and are postulated to lead to several adverse gynaecological outcomes.3–5 This has been hypothesised to be due to micro-trauma, inflammation and changes to the vaginal pH and vaginal flora thereby interfering with protective immunological mechanisms.6–8 However, evidence to support this association has been conflicting. Most recently, the association of IVPs with human immunodeficiency virus (HIV) was investigated in two systematic reviews4,9 and was found to be inconclusive but potentially related to harm. No previous systematic review has assessed the general association of IVPs with human papillomavirus (HPV) and the development of cervical cancer.
Cervical cancer is a major cause of morbidity and mortality in women worldwide, particularly in sub-Saharan Africa and Central and South America.10 HPV infection as the necessary cause of cervical cancer, follows a well-understood pathway to progression, through infection and persistence of high-risk subtypes, to pre-cancerous changes and subsequent invasive cervical cancer.11 The initiation of HPV infection is thought to be facilitated by micro-trauma in the cervical epithelium.12 Some factors have been associated with an elevated cervical cancer risk in women with HPV-infection, such as long-term oral contraceptive use, smoking and other sexually transmitted infection (STI) co-infection.13 Additionally, HIV is recognised as a significant contributor to cervical cancer progression due to immunosuppression.14,15 As IVPs are often described in regions with high prevalence of HIV, HPV infection and cervical cancer, it is critical to continue to ascertain the possibility of risk associated with use of IVPs and HIV infection. These potential associations are illustrated (Figure 1).
We describe a systematic review to assess the evidence of an association of IVPs with HPV infection, and the development of cervical cancer, using ‘cervical disease’ as an overarching term to encompass pre-cancerous changes and cervical cancer.
Objectives
In order to examine whether intravaginal practices are associated with HPV infection and cervical cancer development, we systematically reviewed primary observational studies covering a range of IVPs in different geographical regions and their associations with HPV infection and cervical disease. Additionally, we examined recent evidence describing any association of IVPs with HIV infection.
METHODS
Protocol and registration
A protocol was developed using the Preferred Reporting Items for Systematic Review and Meta-Analysis-Protocol (PRISMA-P) guidelines16 and is provided in Online Supplementary Document.
Eligibility criteria
Studies eligible for inclusion were cohort, case-control and cross-sectional studies, investigating the relationship between intravaginal practices in women of all ages in any global setting and cervical HPV, cervical disease or HIV infection. A broad definition of IVPs was adopted, taking the practices as defined by the individual study authors but excluding practices that were only external or were not self-administered. Where IVPs were examined as part of a risk factor profile, studies were eligible provided IVPs were analysed separately with effect measures given. No restrictions were placed on the purpose for conducting IVPs. Any studies that considered sexually transmitted infections in general without the specific mention of HPV or HIV infections were excluded. No location or language were specified to ensure the range of geographical variation was captured. In relation to date of publication, only studies published after 1990 were considered for HPV and only after 2008 for HIV due to the prior systematic reviews on the topic including studies published prior to this date. Both published and unpublished studies were eligible for inclusion.
Literature searches
A comprehensive search strategy, devised with the help of a University of Edinburgh health sciences librarian, was adapted for use in six electronic databases, namely MEDLINE (Ovid Interface), EMBASE (Ovid Interface), Global Health (Ovid Interface), Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCOhost), POPLINE (Population Information Online) and Web of Science, from 01 January 1990 to the 03 June 2019. Additionally, grey literature sources including OpenGrey, the Grey Literature Report, the WHO African Region Library and ProQuest Dissertations and Theses were searched and the reference lists of all full-texts for inclusion were scanned to identify any other relevant studies. Briefly, search terms were a combination of Medical Subject Headings (MeSH) and free text terms based on variations of the exposure and outcome of interest; namely ‘intravaginal practice’, ‘vaginal douching’, ‘intravaginal insertion’, ‘intravaginal cleansing’, ‘hygiene’, ‘application’, ‘modification’, ‘dry sex’, ‘human papillomavirus’, ‘cervical dysplasia’, ‘human immunodeficiency virus’ and ‘sexually transmitted infection’. The detailed search strategy is available in Online Supplementary Document.
Study selection
References were imported into EndNote Reference Manager X8 (Clarivate Analytics, Philadelphia PA, USA) and titles and abstracts were screened independently by two reviewers (TM and RH). Any discrepancies were resolved by discussion. The same method was used for full-text screening.
Data collection process
Using a data extraction form designed using guidelines set out by Cochrane17 and subsequently piloted on five studies and revised, data was extracted by a single reviewer (TM) and checked by a second (RH). Data was extracted on study authors, study design, year of publication and conduct of the study - the country, number and characteristics of study participants, the definition of intravaginal practices as described in the study, length of follow-up and timing if applicable and the outcome measures used in the study including the method of HPV diagnosis and results as given by effect measures.
Quality assessment
The Appraisal Tool for Cross-Sectional Studies (AXIS) and the Newcastle-Ottawa Scale (NOS) for cohort and case-control studies were used to assess quality.18,19 This was undertaken by one reviewer (TM) and checked by a second (RH), with discussion for consensus. However, studies were included in the analysis regardless of the quality, with consideration of the limitation on the strength of the conclusions drawn.
Planned methods of analysis
The marked heterogeneity due to differing definitions of intravaginal practices, variations in study design, participant characteristics and measurement of outcomes precluded undertaking a meta-analysis. Therefore, we carried out a narrative synthesis according to guidelines set out by Popay et al.20 Common patterns across studies were combined and components that could potentially have a bearing on risk were evaluated. Where there were both conference abstracts and full-text publications available from a study, the reported findings were drawn from the latter. One conference abstract21 was included as an additional study but was not included in the analysis as the full-text publication was not available after contacting the study author.
Risk of bias across studies
The weight of evidence cumulatively was assessed using the Evidence for Policy and Practice Information and Coordinating Centre (EPPI) Weight of Evidence Approach through the application of individual weights based on the methodological robustness, appropriateness of study design and relevance.22 An overall weighting for each study was assigned by one reviewer (TM) according to the combination of all three. This was checked by a second reviewer (RH), with discussion for consensus, and is presented in Online Supplementary Document.
Ethics
As no primary data collection was undertaken, formal ethical approval was not required. A study self-audit was submitted to the Usher Institute Ethics Committee at the University of Edinburgh.
RESULTS
Study selection
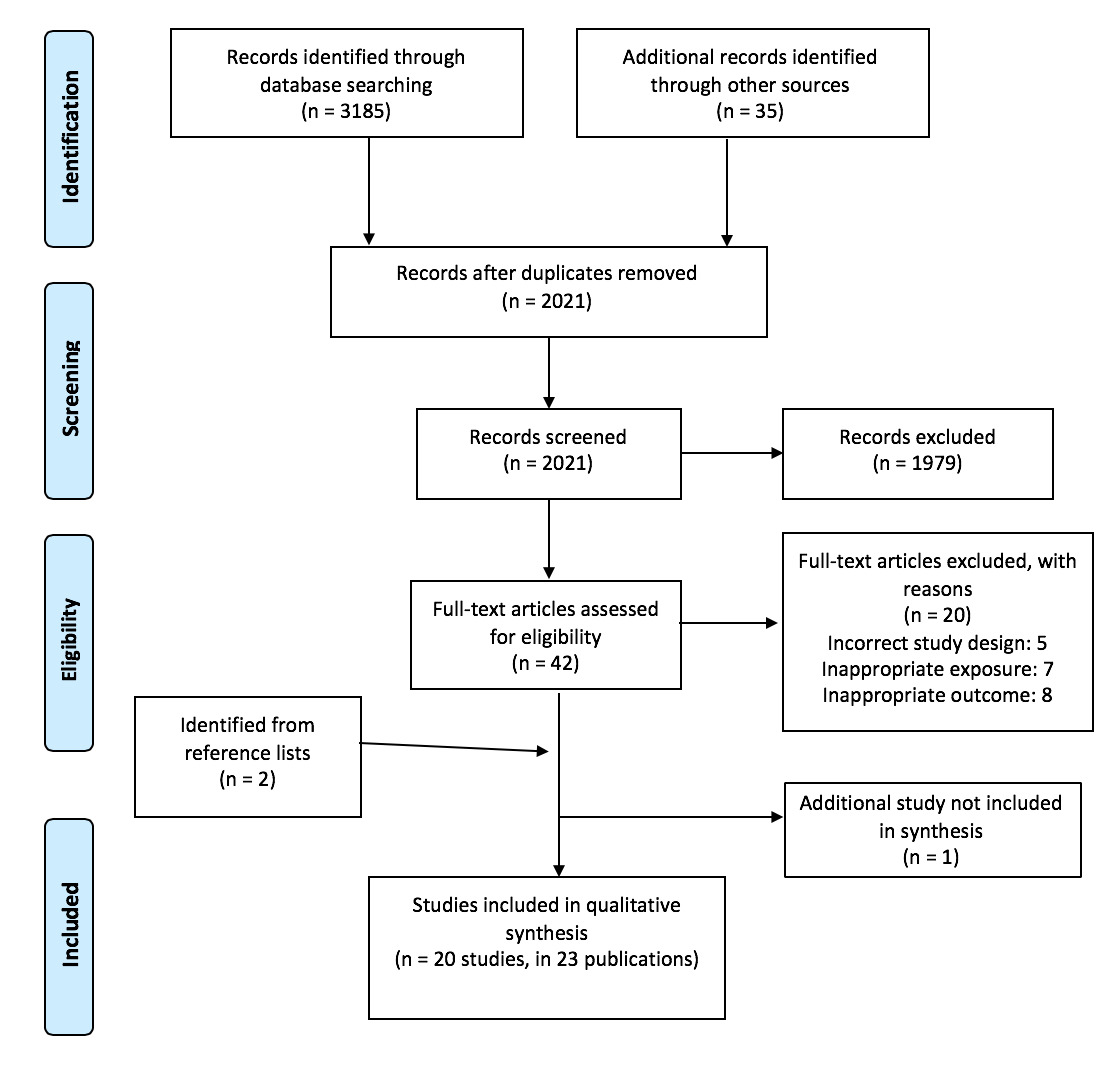
Figure 2 shows the PRISMA diagram, illustrating the study selection. An initial 3,185 records were identified, of which 2,021 remained after de-duplication. After title and abstract screening, 42 records underwent full-text screening: 18 studies in 21 records were identified as eligible for inclusion. A further two studies were identified through scanning the reference lists of included studies, leading to a total of 20 studies in 23 records for analysis. A full list of excluded studies and reasons for exclusion is provided in Online Supplementary Document.
Study characteristics
The characteristics of individual studies are described in Table 1, categorised according to outcome. Thirteen studies were cross-sectional,23–35 five were cohort studies36–40 and two were case-control studies,41,42 published between 1991 and 2018. In total, they accounted for 14,493 participants. Nine studies were carried out in sub-Saharan Africa (Democratic Republic of the Congo (DRC), Malawi, Mali, Nigeria, South Africa, Tanzania, Uganda, Zimbabwe), five in Asia (Cambodia, China, South Korea, Taiwan), four in the United States of America (USA), one in Haiti and one in Brazil. The majority (12 studies) included participants recruited from health care settings such as clinics and hospitals,28,32–42 and four23,25–27 were based on female sex worker (FSW) cohorts. One study included adolescent participants before reported sexual debut.29
IVPs described varied in description, detail and substances used (if recorded). Douching was most commonly studied, analysed in ten studies, with three from Asia,30,32,37 three from the USA,33,38,41 two from sub-Saharan Africa,25,42 one from Haiti31 and one from Brazil.40 However, a definition in keeping with that proposed by the WHO GSVP1 was only provided in one study42 while the others solely stated douching as the IVP under investigation. Two other studies which were reportedly evaluating douching had definitions more aligned with general intravaginal cleansing and were hence analysed with this group.24,26 Intravaginal cleansing and intravaginal insertion were heavily skewed towards sub-Saharan Africa, described in seven studies from this region27–29,34–36,39 and in one additional study from Cambodia.23
Substances were frequently water-based with the addition of natural and commercial products such as salt, lemon or lime juice, vinegar, plant-based agents, medical disinfectant, toothpaste and soap.23,25,26,28,29,34,37 The purpose for conducting IVPs where recorded was cited as hygiene most frequently, both for general cleanliness and around sexual intercourse.26,27,41 Other reasons were for infection prevention or management and for contraception.25–27,41 Intravaginal insertion was practised using herbs acquired from traditional healers, alum, tobacco and other powders, castor oil and medications, with the main purpose being for drying, tightening and warming the vagina prior to sexual intercourse.27–29,36,39 The method of application was only mentioned in three studies, commonly the use of fingers, cotton wool or cloth.28,29,36
Studies assessed IVPs in relation to the three outcomes of interest; 11 examined HPV infection, seven examined cervical disease and two examined HIV infection, in all cases either as the primary focus or as part of an assessment of multiple risk factors.
Quality assessment
Using the AXIS tool, which comprises a checklist of 20-items for overall assessment without the assignment of a score, two cross-sectional studies were assessed as high quality,27,31 seven as moderate23,24,26,28–30,34 and four as low quality.25,32,33,35 Using the NOS, the included case-control studies were both assessed as moderate quality.41,42 Two cohort studies were assessed as high quality37,38 and three were moderate.36,39,40 Detailed quality assessments are provided in Online Supplementary Document.
Intravaginal practices and HPV infection
Eleven studies examined the association between IVPs and HPV infection, of which six assessed douching30–33,38,40 and five assessed intravaginal cleansing and insertion (Table 2).23,24,28,29,39 A clear definition of the IVP was only given in five studies.23,24,28,29,31 The prevalence of intravaginal insertion was much less than that observed for intravaginal cleansing, ranging from 0.2–32.1% and 20.9–94% respectively,23,29,39 while that of douching ranged from 41.3–97.1%.31,33 Most studies focused on the likelihood of HPV detection and prevalence, which ranged from 7.9% in a South Korean study to 76.3% in a study conducted in South Africa,30,39 with the exception of one study assessing the likelihood of HPV redetection after documented clearance.38
Six studies demonstrated evidence of a harmful association, with the greatest effect measure recorded from a study conducted in Haiti with an adjusted odds ratio (OR) of 5.01 (95% CI 1.56–16.05), between douching with a pigeon pea infusion and HPV infection.31 Four found moderate associations, with one finding that douching was significantly associated with infection with a higher number of HPV subtypes,24 and the remaining three reported associations with increased risk of HPV infection and prevalence.29,32,33 Douching was also associated with a higher likelihood of HPV type-16 (high-risk subtype) redetection.38 One study showed an association between IVPs and HPV prior to sexual debut (OR 2.19, 95% CI 1.09–4.39), although the authors proposed that this might be due to underreporting of self-reported sexual activity.29
Protective associations were suggested in two studies: one found frequent douching was associated with a lower prevalence of HPV infection; the other found that regular intravaginal washing, especially when occurring post-coital was associated with infection with lower numbers of HPV subtypes.23,30 There was no evidence to support an association between douching, intravaginal cleansing or insertion and HPV infection in two studies.28,40 The negative finding for intravaginal insertion may have been due to the low prevalence reported in the study populations; 5% in Malawi and 5.5% in Cambodia, which were likely underpowered to detect associations.23,28
Intravaginal practices and cervical disease
Cervical disease was assessed on the spectrum from low- to high-grade dysplasia and subsequent invasive cervical carcinoma, with seven studies examining associations with IVPs (Table 3), of which three provided clear definitions of the IVPs under investigation.34,36,42 Four studies assessed douching25,37,41,42 and three assessed intravaginal cleansing and insertion.34–36 The prevalence of cervical changes varied from 6.1% in Taiwan37 to 24.6% in Nigeria.25
Of the four studies evaluating douching, three found this practice to be associated with a higher risk of cervical disease. One study carried out among a female sex worker (FSW) cohort in Nigeria found that although vaginal douching with lemon or lime juice was significantly associated with the detection of high-grade squamous intraepithelial lesion (HSIL) (OR 2.13, 95% CI 1.03–4.40, P=0.025), the strength of this association lessened when combined with low-grade squamous intraepithelial lesion (LSIL) (OR 1.76, 95% CI 1.0–3.10, P=0.042).25 In a study examining douching and carried out in the USA, douching frequency (>4 times/month) was found to be associated with increased risk of in-situ and invasive cervical carcinoma (adjusted OR 4.7, 95% CI 1.9–11).41 In another study, carried out in Taiwan, although douching after sexual intercourse was found to be associated with the non-regression of LSIL, this was not maintained as a risk factor for progression.37
Two out of three studies found evidence of a harmful association between intravaginal cleansing and insertion, and cervical disease. Use of herbs for intravaginal insertion in Zimbabwe was found to be associated with cervical dyskaryosis (aOR 2.16, 95%CI 1.18–4.61).35 Similarly, insertion of plant products was associated with LSIL or poorer outcomes in a study in the DRC (aOR 2.70, 95% CI 1.04–7.01).34 A third study, although suggestive of a positive association for the use of finger cleansing, intravaginal wiping and inserting traditional substances with dysplasia on Pap smear, did not reach significance in adjusted analyses (RR 2.42, 95%CI 1.00–5.90, P=0.050).36 Use of IVPs was almost universal in this study population, which may have limited the ability to detect associations.36
Intravaginal practices and HIV infection
We identified two studies that examined the association between IVPs and HIV infection (Table 4), both of which provided clear definitions of IVPs. One was carried out in China among female sex workers, the other in Uganda among women involved in high-risk sexual behaviours.26,27 Weak positive associations were demonstrated between intravaginal cleansing and HIV prevalence in both studies, finding adjusted odds ratios of 2.29 (95%CI 1.01–5.23) and 1.32 (95%CI 1.00–1.73), P=0.05 respectively.26,27 The use of toothpaste and medical disinfectant were the only statistically significant associations.26 Similarly, this was observed for intravaginal cleansing with soap in the Ugandan study.27
We examined whether or not HIV infection was explored in the studies investigating associations between IVPs with HPV and cervical disease (Online Supplementary Document.): less than half the studies stratified by HIV status; none found a significant relationship.
Potential moderator variables
We examined potential moderator variables for association of IVPs with the outcomes of interest (Table 5).
Seven studies assessing association with HPV infection described the substances used,23,28,29,31,32,39,40 with all except one,39 further analysing associations with each of the substances separately. A positive association was demonstrated in two studies:31,32 in one, normal saline or detergent use for douching was found to be more harmful than tap water in adjusted analyses;32 in the other, an association between douching and a higher risk of HPV infection was found with a pigeon pea infusion.31 Four studies did not find statistically significant associations between the substances used for douching, intravaginal cleansing or intravaginal insertion and the risk of HPV infection.23,28,29,40 The frequency of IVP use was examined in seven studies,23,24,28–30,33,40 with three24,29,33 reporting a positive dose-response effect: the higher the frequency of the IVP use, the higher the likelihood of HPV infection. The remaining studies28,30,40 did not demonstrate an association between increasing frequency of IVP use and the likelihood of HPV infection, but one found that the highest frequency of douching was protective for HPV infection.30
In terms of potential moderators for an association of IVP use and cervical disease, only three studies34,37,41 analysed by the specific IVP substance used. One study37 found higher odds of LSIL non-regression with saline or detergent (OR 3.14, 95% CI 1.04–9.49) compared to tap water (OR 0.77, 95% CI 0.94–3.35). Another,41 comparison of chemical and commercial solutions, water alone, and water in combination with vinegar or soda found risk did not depend on the substance used.41 In the third study, although the use of chemicals was more prevalent than plant products (26% vs. 11%), only plant products had a significant association with the presence of LSIL or more severe lesions, with an adjusted odds ratio of 2.70, 95% CI 1.04–7.01.34 Two studies25,37 reported on timing of IVP use. Post-coital vaginal douching was found to be associated with harm, but no comparisons were made with pre-coital timing.37 The second study mentioning timing did not analyse pre-coital and post-coital timing separately and instead considered douching as a whole.25 Two studies commented on the duration for which IVPs were used; a study evaluating an FSW cohort noted that although douching with lemon or lime juice was closely associated with the duration for which participants had been employed in commercial sex work, this did not seem to have an impact on the likelihood of cervical disease;25 the other study,36 although reporting on duration did not analyse its impact on the associations.
DISCUSSION
Summary of findings
Overall, the current evidence base does not allow firm conclusions to be drawn on the association between IVPs, HPV infection or the development of cervical cancer, although there is a broad suggestion of harm. This systematic review included 20 studies in 23 publications of which most were of low to moderate quality. As most studies involved douching, drawing associations between the outcomes of interest and other types of IVPs was limited.
The prevalence of IVPs was high in most study populations, but with wide variation in the specific practice, and by geographic location. Douching or intravaginal cleansing were much more common than intravaginal insertion in general, reflecting findings from previous studies.43–45 Eleven out of 18 studies reported an elevated risk of HPV infection, or cervical disease, with IVP use. However, it was not possible to draw firm conclusions of association between HPV infection and either intravaginal cleansing or insertion, similar to findings demonstrated in relation to HIV infection.4 In contrast, there is some evidence of increased risk of cervical disease, with both intravaginal cleansing and insertion.
Strengths and limitations of the study
The main strengths of our review included the comprehensive search strategy, and searching of six databases as well as grey literature sources. A diverse range of studies covered varying geographical locations, with the inclusion of over 14,000 participants, and good representation of women from the general population as well as female sex workers. Quality assessment was carried out using validated tools.
Limitations to our systematic review are important to note. At present, most evidence lies in cross-sectional studies and therefore the detection of HPV infection was only limited to recently acquired infections which may have been transient. The inability to establish temporality limited clear understanding of the possible mechanism of association as the persistence of HPV infection is a pre-requisite for progression to cervical cancer.12 The substantial heterogeneity brought about by the absence of use of clear definitions for IVPs and detail regarding their specific application also limits an understanding of the potential association. In addition, there is the possibility of attenuated risk occurring where multiple IVPs were undertaken in the same study population, but it was not possible to disaggregate data. Douching was the most frequently studied practice, with limited data on intravaginal cleansing practices or intravaginal insertion: reporting of these may be influenced by social desirability bias. Although the geographical diversity captured is recognised as a strength and similarities were noted across different countries, it is important to acknowledge that certain context-specific nuances with respect to IVPs may not have been comprehensively reflected, given their culturally engrained nature46.
Existing literature
We have identified no previous systematic reviews on IVP use and risk of cervical disease: a meta-analysis from 1997 examined the relationship between douching and cervical cancer and found a marginal association.3 That study reported pooled results from six case-control studies with invasive cervical cancer as the outcome and suggested that associations could be related to the preparation used for douching, as well as the frequency and timing.3 However, due to the length of time required for the development of cervical cancer, we instead considered possible associations between the acquisition of HPV infection, and subsequent progression to cervical cancer, with a variety of IVPs in addition to douching. Exploration of the effect of potential moderator variables was limited in our review due to the lack of detail in reporting in the primary studies and findings were mixed. The specific substance used for IVPs could possibly contribute to a higher risk of HPV infection or changes leading to persistence and cervical cancer development through the chemical content or degree of abrasiveness.3,5,47 In our review, nine studies analysed this variable: four showed evidence of an increased risk. However, there was considerable heterogeneity in the substances assessed, from normal saline, toothpaste and disinfectants to various plant and commercial products.
Similarly, the frequency of undertaking IVPs was inconclusive, likely contributed to by the inconsistencies in definitions for ‘frequent’ and ‘infrequent’, with some measuring daily frequency and others assessing conduct of IVPs over a weekly or monthly basis. The frequency of use had been hypothesised to be an important factor in relation to douching and the acquisition of bacterial STIs and other negative outcomes, possibly due to the decreased time for recovery of the vaginal flora with more frequent conduct of IVPs.48 Other factors, such as timing and duration of use of IVPs could not be clearly assessed as they were not consistently reported in the included studies.
There is limited new evidence of association of IVPs and HIV infection since 2008, with the identification of only two full-text studies. Consistent with previous findings,9 both studies reported harm associated with intravaginal cleansing,26,27 but one reported no association with intravaginal insertion.27 A conference abstract (excluded at full-text stage), suggests decreased risk of HIV infection with intravaginal cleansing.21
Implications
There is a need for prospective longitudinal studies in populations where IVPs are prevalent, to better understand whether and how IVPs may affect the timeline of progression to cervical cancer, or other gynaecological outcomes. Given that most evidence lies in low to moderate quality studies, future studies should include larger and more representative samples. In addition, consistent use of standardised definitions and categorisations of IVPs, such as those proposed by the WHO GSVP study group, would enable clearer comparisons between populations and regions.1 It is essential to limit the effects of social desirability and recall bias in reporting, using contextually and culturally appropriate methods to elicit understanding and reporting of the types of IVPs (such as the self-administered pictorial diary used in Uganda and Tanzania).44
Exploration of mediating factors for risk should also be improved. An association between HIV infection and IVPs has been proposed to be related to an increased risk of abnormal flora, in particular bacterial vaginosis which could then facilitate transmission, rather than a direct linkage.4,9 Bacterial vaginosis has been investigated in two prior systematic reviews exploring associations with HPV infection as well as with cervical intraepithelial neoplasia.49,50 However, these studies did not investigate IVPs, so there remains scope for investigation of this and other potential mediators.
Although the precise mechanism remains unclear, the extent of the evidence suggestive of harm in the included studies in this review is a cause for concern. In keeping with previous studies, a high prevalence of IVPs was described in most of the included studies, although this differed depending on the practice described. With the majority of participants in the included studies recruited from health care facilities, it has been proposed that health professionals may be well positioned to ascertain whether IVPs are utilised and to promote education and awareness about their possible risk.51–53 Some scholars have proposed group delivered interventions for peer support and motivation to facilitate discussion, and possibly cessation, of IVPs may be effective.54,55
Health education interventions about the potential risk of IVPs have often been implemented in the context of HIV infection,53,56 where the importance of using culturally sensitive and acceptable approaches are well-recognised. This approach could be further adapted to include messages about HPV infection and cervical cancer development. Given that IVPs tend to be socially and culturally engrained, sensitivity is required in understanding their motivating and perpetuating factors and the beliefs surrounding their benefits as well as any cervical cancer risk.5,52,56 In addition, a clear distinction should be made between evidence-based practices that may involve intravaginal insertion, such as the clinical use of vaginal suppositories for treating infection and contraceptive vaginal rings57,58 from the potentially more harmful insertion practices described in this review. This issue has been a source of concern in some study populations, amongst whom beliefs exist that any product applied intravaginally, even if medically indicated, could be harmful.52 It will be important to promote clear public health advice on this topic going forwards, particularly with the potential advent of new evidence-based intravaginal devices such as antiretroviral intravaginal rings for HIV pre-exposure prophylaxis which have been undergoing clinical trials.59–61
CONCLUSIONS
The ability to draw firm conclusions regarding an association of IVPs with HPV infection, or the development of cervical cancer, was limited by the current evidence base. Although the possibility of harm was suggested overall, IVPs were not clearly defined in the identified studies and there was limited capacity to assess mediators of the pathway. It may be necessary to increase awareness related to potential risks of IVPs and to conduct these efforts with cultural sensitivity, but this must be substantiated by more robust evidence to ensure that clear and consistent messages are promoted.
Acknowledgements
We thank Marshall Dozier, Academic Support Librarian at the University of Edinburgh for invaluable advice on search strategies; we also thank Liz Grant and Cecile Wabnitz for early discussion of the interface of IVPs and cervical disease.
Funding
This study had no external funding.
Authorship contributions
All authors meet authorship criteria. CC and TM conceived the study; TM led the protocol development and literature searching, and initial data extraction and quality assessment, and data synthesis; RH was the second reviewer. All authors contributed to interpretation of findings, and contributed to the writing of the paper. CC is the guarantor.
Competing interests
The authors completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author), and declare no conflicts of interest.
Correspondence to:
Tafadziswa T Museba
Usher Institute, University of Edinburgh, Teviot Place, Edinburgh, EH8 9AG, United Kingdom.