Increased access to antiretroviral therapy (ART) for people living with HIV (PLWH) has substantially improved survival in countries with a high HIV prevalence.1 In Uganda, ART coverage reached 70% by 20172 while life expectancy at birth increased from 45 years in 2004 (the year ART services were initially rolled out) to 62 years in 2017.3 In the meantime, there has been an increase in the burden of noncommunicable diseases (NCDs) in most of sub-Saharan Africa.4 With better survival and subsequent ageing, PLWH are faced with the increasing risk of NCDs,5,6 predominantly cardiovascular diseases (CVD).7 This has partly been attributed to inflammatory processes in HIV infection that are associated with an increased risk for CVD.8 In addition, as a result of increased ART coverage, PLWH increasingly have similar cardiometabolic risk profiles as those of HIV-uninfected individuals.9
Several studies in high-income countries found a higher CVD risk in PLWH compared with HIV-uninfected individuals.7 A pooled analysis of these studies estimated a 79% increased risk for myocardial infarction due to HIV infection and more than double the risk for stroke.7 In sub-Saharan Africa, studies comparing CVD risk by HIV status remain limited. Most studies are cross-sectional comparisons of traditional CVD risk factors by HIV status and have concluded that PLWH have more favorable CVD risk profiles.9 A systematic review estimated that PLWH in sub-Saharan Africa have on average 2·3 kg/m2 lower body mass index (BMI) than HIV-uninfected individuals, and their systolic blood pressure is on average 5·6 mmHg lower.9 Longitudinal studies assessing CVD events are also sparse in sub-Saharan Africa due to challenges establishing these cohorts and in documenting CVD events. A case-control study from Tanzania found that HIV infection was associated with five times higher odds of stroke.10
Furthermore, national estimates on the prevalence of CVD risk factors and CVD risk in PLWH are not available in most of sub-Saharan Africa where countries do not have nationally representative surveys that jointly assess HIV/ART status and cardiometabolic risk factors and lack complete vital registration systems. In the absence of primary data on hard CVD endpoints, risk prediction models are a practical alternative but most of these models were developed using data from high-income countries that are not applicable to PLWH in sub-Saharan Africa. In addition, most models do not account for the direct effect of HIV on CVD,8 an effect that is independent of changes in risk factors like blood pressure, diabetes and total cholesterol. Excluding this effect would potentially underestimate CVD risk among HIV-infected individuals as was shown in a Ugandan study that predicted lower CVD risk in PLWH than in matched HIV-uninfected controls.11 This comparison was based on predictions made with Framingham and American Heart Association’s pooled cohort risk scores, neither of which include this direct effect. Other studies have used the Data collection on Adverse Effects of Anti-HIV Drugs Study (D:A:D) prediction model which takes into account the effects of low immunity (CD4 cell count) and specific ART drugs on CVD risk.11,12 However, the D:A:D model was also developed for high-income countries and has not been recalibrated for use in sub-Saharan Africa.13
To fill this gap, we developed a microsimulation model to quantify the proportion of PLWH in Uganda with raised blood pressure and high total cholesterol, and used a risk prediction model calibrated for Uganda that takes into account the direct effect of HIV on CVD, to predict the burden of atherosclerotic CVD (stroke and coronary heart disease) among PLWH.
Methods
We used various data sources to create a nationally representative sample of Ugandans aged 30 to 69 years, with estimates on traditional CVD risk factors (systolic blood pressure, diastolic blood pressure, total cholesterol, diabetes and smoking), as well as HIV and ART status. We then quantified the proportion of individuals with raised blood pressure and high total cholesterol using standard clinical thresholds. Thereafter, we used values on systolic blood pressure, total cholesterol, diabetes, smoking status, as well as age, sex and HIV status to predict each individual’s 10-year risk for fatal and non-fatal CVD.
Data sources
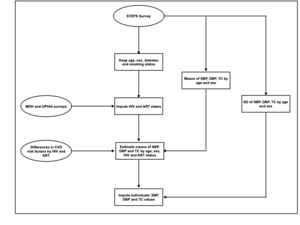
Individual-level values on systolic blood pressure, diastolic blood pressure, total cholesterol, diabetes and smoking were available from the nationally representative 2014 Uganda STEPS Survey.14 Age and sex-disaggregated estimates for HIV prevalence were taken from the 2016 Uganda Population Based HIV Impact Assessment Survey (supplementary Table S1).15 We derived ART coverage estimates by sex for 2016 from a report by Uganda’s Ministry of Health.16
Effect of HIV and ART on CVD risk factors
HIV infection and ART have both been associated with changes in traditional CVD risk factors.9 We identified from a pooled analysis of sub-Saharan African studies, the mean differences in systolic blood pressure, diastolic blood pressure and fasting blood glucose by HIV and ART status.9 We updated findings of this pooled analysis by including recently published articles (Table 1).
Creating a nationally representative sample
We created a nationally representative sample of adults aged 30 to 69 years with individual-level data on HIV/ART status and on traditional CVD risk factors. This enabled us to take into account the direct effect of HIV on CVD, as well as the effects of HIV and ART on CVD that are mediated through traditional CVD risk factors. We included individuals aged 30 to 39 years because CVD events have been observed to occur at younger ages in HIV-infected individuals17; we did not include individuals older than 69 years because this age group represents a small population in Uganda (1% in 2016)15 and was not included in the STEPS survey. As HIV status is not assessed in the STEPS survey, we used data on HIV prevalence and ART coverage to impute each individual’s HIV and ART status by five-year age groups and sex, to create three categories of individuals: HIV-uninfected, HIV-infected not on ART, and HIV-infected on ART. We thereafter used data on the distributions of traditional CVD risk factors in the STEPS survey by age group and sex, mean differences of these risk factors by HIV and ART status (Table 1), as well as estimates for HIV prevalence and ART coverage to impute each individual’s systolic blood pressure, diastolic blood pressure and total cholesterol levels. We kept the individual-level observed values from the STEPS survey for diabetes and smoking as there is no evidence for an effect of HIV, or ART on these two risk factors in Uganda (Figure 1).
Estimating CVD risk and CVD events in HIV-infected and HIV-uninfected individuals
We modified the Globorisk model which was developed to estimate 10-year risk of stroke and coronary heart disease globally.18 This model uses pooled data from eight large prospective cohorts in the US and can be recalibrated for use in other countries. For Uganda, this was done by using age- and sex-specific CVD rates, and average CVD risk factor levels in the population (from the STEPS survey). We used CVD rates in Uganda from the World Health Organization (WHO)18 that are comparable to those of the Global Burden of Disease (GBD) study19 (Online Supplementary Document, Figure S1). We modified the risk prediction equation to include the direct effect of HIV on CVD that is not mediated through traditional CVD risk factors (Online Supplementary Document, Figure S2). To quantify the direct effect, we re-analyzed studies included in a recent meta-analysis.20 We identified studies in this meta-analysis with estimates of effect of HIV on CVD that is not mediated through metabolic risk factors, and pooled them separately for coronary heart disease and stroke. We combined the two pooled hazard ratios (HR) using the incidence rate of coronary heart disease and stroke in Uganda per the most recent estimates of the GBD study19 as weights to calculate a single HR for the direct effect of HIV on CVD (HR=1·63, 95% confidence interval, CI=1·31-1·93). The modified model that includes the direct effect of HIV is:
φi(t)=φ0,s(t)exp[ 4∑l=1βl(Xl,i−¯Xl,s,t)+ 4∑l=1δlt(Xl,i−¯Xl,s,t)+ 2∑l=1γlsexi(Xl,i−¯Xl,s,t) +α(Hi−¯Hs,t) ],(1)
where is the CVD rate for individual of HIV status at age is the age- and sex-specific CVD rate; denotes CVD risk factors in the model (systolic blood pressure, total serum cholesterol, diabetes, and smoking); is the log hazard ratio of each risk factor from Globorisk18; are mean levels of risk factor for each age-sex and HIV status; represents the interaction between age and risk factor and represents the interaction term between diabetes/smoking and sex; represents the direct effect of HIV on CVD (in log relative risk scale); and is the age-sex-specific prevalence of HIV. We assume a constant risk factor profile over the ten-year prediction period to estimate the CVD risk for an individual aged as:
Pi(CVD)=1−t+9∏j=t(exp[−φi(j)]),(2)
where is the individual’s age at each of the subsequent nine years, and is defined in (1).
We compared the estimated CVD risk under ART coverage levels of 2016 to a hypothetical scenario where all HIV-infected individuals would be on ART (i.e. full coverage). Next, we assessed the distribution of fatal and non-fatal CVD risk in the population by sex and HIV status. Following the American College of Cardiology/ American Heart Association’s (ACC/AHA) criteria,21 we used a 7·5% 10-year CVD risk threshold to identify high-risk individuals that would benefit from using statins for primary prevention of CVD. To quantify the number of CVD events for each age (30 to 44, 45 to 59, and 60 to 69 years) and sex group, we multiplied its mean CVD risk with the size of the corresponding population using estimates from the United Nations Population Division (Online Supplementary Document, Figure S3).22
To incorporate uncertainty in our modeling, we ran n=1000 simulations and extracted the 2.5, 50 and 97.5 percentile values for the point estimate and the confidence bounds. In each simulation, we extracted values from independent distributions of the following input parameters: cardiometabolic risk factors, their mean differences by HIV and ART status, HIV prevalence, and the HR for the direct effect of HIV on CVD. All analyses were performed using Stata version 15 (Stata Corp., TX, USA).
Ethics approval
No informed consent was required because study procedures did not involve research on human subjects. The study was approved by the ethical review boards of Mbarara University of Science and Technology (# MUREC 14/09-17), the Uganda National Council of Science and Technology, and the Harvard Chan School of Public Health (IRB16-2062).
Results
A total of 6,975,373 adults aged 30-69 years were included in the analysis and a majority (58%) were women. The prevalence of HIV was 11% in women and 9% in men, and ART coverage was 77% for women and 54% for men.
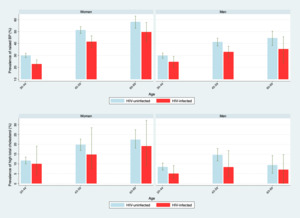
The estimated mean systolic blood pressure in women was 129 mmHg in those who were HIV-uninfected and 122 mm Hg in HIV-infected individuals. For men, mean systolic blood pressure was 129 mm Hg in those who were HIV-uninfected and 123 mmHg for HIV-infected counterparts. HIV infection was associated with a lower prevalence of raised blood pressure (Figure 2). The estimated mean total cholesterol levels were generally low, more so in men. The mean total cholesterol level in HIV-infected women was 4·0 mmol/L, and 3·9 mmol/L in women who were HIV-infected. For men, the mean total cholesterol level was 3·8 mmol/L in those who were HIV-uninfected men, and 3·5 mmol/L in HIV-infected individuals (Online Supplementary Document, Table S2).
The predicted mean 10-year risk for CVD was 5.0% in HIV-infected women, 3.5% in HIV-uninfected women, 6.3 % in HIV-infected men and 4.7% in HIV-uninfected men (Figure 3, panel A). Although HIV infection led to a higher estimated 10-year risk of CVD (Figure 3, panel A), a large proportion of HIV-infected individuals were at low risk for CVD as shown by a mean 10-year CVD risk of 5% in women and 6% for men. 19% of HIV-infected women and 28% of HIV-infected men would meet criteria for primary prevention of CVD according to ACC/AHA guidelines (Figure 3, panel B). Our model predicted about 41,000 CVD cases to occur from 2016 to 2025 among HIV-infected individuals (19,000 in women and 22,000 in men), and about 320,000 cases among HIV-uninfected individuals (140,000 in women and 180,000 in men, Figure 4), a reflection of the respective numbers of HIV-infected and HIV-uninfected individuals in Uganda. Thirty-eight percent of the predicted CVD cases in HIV-infected individuals were fatal (44% in women; 33% in men). Among HIV-infected women, the predicted number of CVD cases was on average similar across the three age groups (ranging from 5,400 to 7,800), a pattern that is explained by the prevalence of HIV, the CVD rate and the population from each specific age group. For example, although HIV prevalence and population sizes were highest among women aged 30 to 44 years, higher CVD rates in women of older age groups resulted in almost similar CVD cases predicted for the three age groups. A different pattern was predicted for HIV-infected men: CVD cases doubled from 6,000 cases in men aged 30 to 44 years to over 12,000 cases in those aged 45 to 59 years because of the higher prevalence of HIV in older men (5·6% in men aged 30 to 34 years vs. 14·0% in those aged 45 to 49 years) and a higher CVD rate in older age groups. The predicted number of CVD cases subsequently declined to 3,000 cases in HIV-infected men aged 60 to 69 years due to the small population figures in this age group. Full ART coverage would have little impact on CVD risk due to already high ART coverage levels in 2016. For example, full ART coverage in 60 to 69 year-olds would result in a similar 10-year risk for CVD to the one that was predicted for ART coverage levels of 2016 (17% vs. 16% for women, and 18% vs. 20% for men). The predicted number of CVD cases over ten years would also remain fairly unchanged (Online Supplementary Document, Figure S4).
Discussion
We estimated the burden of CVD among PLWH in Uganda. Overall, we found that HIV infection was associated with a lower prevalence of raised blood pressure but a higher 10-year risk for CVD due to the harmful direct effect of HIV on CVD risk. Our findings further highlight that despite the higher risk of CVD in PLWH, the predicted burden of CVD is still low among this population with 5% (41,000) of all cases of stroke and CHD estimated to occur among PLWH from 2016 to 2025. To our knowledge, this is one of the first studies that uses a CVD risk prediction model calibrated for a specific sub-Saharan African country, and that includes the direct effect of HIV to quantify the national burden of CVD among PLWH.
Our estimates on traditional CVD risk factors (blood pressure and total cholesterol levels) are consistent with those of a study in western Uganda where PLWH had lower odds of hypertension than matched HIV-uninfected controls but where no difference was observed in mean total cholesterol levels by HIV status.23 Our estimates are representative of the CVD risk profile in Uganda in 2016: the lower proportion of raised blood pressure in HIV-infected individuals is in part due to immunosuppression in those who had not started ART by 2016 (23% of women and 46% of men).16 These observed differences in CVD risk factors by HIV status are likely to reduce as more HIV-infected individuals start ART early. Yet, almost a third of adults living with HIV require treatment for raised blood pressure, a service that might not be readily available in most HIV treatment facilities. As per ACC/AHA guidelines, a sizable proportion (19% of women and 28% of men) would also require statins for primary prevention of CVD, a therapy that is even more difficult to access in Uganda than treatment of raised blood pressure.
The estimated higher risk of CVD among HIV-infected individuals is due to the direct effect of HIV that outweighs the beneficial effect of having lower CVD risk factor levels when compared to HIV-uninfected individuals. We used a direct effect of HIV from a pooled estimate of studies in high-income countries.20 This HR is likely to be conservative because most HIV patients in these studies were on ART, a factor that was linked to lower risk for CVD in the Strategies for Management of Antiretroviral Therapy (SMART) trial.24 Our findings are consistent with previous studies that predicted low mean CVD risk among PLWH in Uganda.11,12 Only 17% of individuals (26% males and 13% females) in one study had a 10-year CVD risk that exceeded 5%.12 However, even slightly higher risk of CVD associated with HIV infection is concerning, particularly in older ages, given the increasing longevity of HIV-infected individuals. In addition, annual new HIV infections in adults are still high in Uganda with over 83,000 new cases reported in 2015.2 A continued trend of a high adult HIV incidence together with effects of aging on CVD risk factors will potentially lead to a larger burden of CVD among PLWH in the future.
The total number of CVD events predicted among HIV-infected and uninfected individuals over ten years (362,000) is comparable to estimates from the Global Burden of Disease study.19 The overall burden of CVD is much higher among HIV-uninfected individuals due to their larger numbers in the population (adult HIV prevalence was 6% in Uganda in 2016; Online Supplementary Document, Figure S3). The number of CVD events in PLWH (about 4,100 cases per year) is smaller than that of common opportunistic conditions in HIV. For example, an estimated 34,000 new cases of tuberculosis (TB) occur among PLWH in Uganda each year.25 The predicted number of CVD cases is also likely to increase as PLWH survive to older ages. Yet, our findings underscore the growing evidence that cardiovascular conditions are a leading non-HIV related cause of death in PLWH in sub-Saharan Africa.5 The high burden of CVD among HIV-uninfected individuals (about 32,000 cases per year) is alarming because the Uganda’s current healthcare delivery system is unlikely to adequately address these large numbers. There has been increased advocacy for building onto existing HIV treatment platforms to deliver care for NCDs to the general population in countries with a high prevalence of HIV.26 There is however limited empirical evidence on how to achieve this, and this NCD-HIV integration approach is also likely to miss the larger burden of NCDs among HIV-uninfected individuals that are not routinely seen at health facilities.
We estimated that increasing ART coverage to 100% would have little impact on the number of CVD cases among PLWH over ten years. This is due to the minimal impact of ART on blood pressure and total cholesterol (Table 1) and the moderately high ART coverage in PLWH in 2016 (77% for women, 54% for men in Uganda) as a result of national policies that have promoted early initiation of ART.16 By 2011, in line with WHO recommendations, the Ministry of Health of Uganda enforced new guidelines requiring ART initiation at higher CD4 cell counts (≥ 350 cells/mm3) than before27; and the test-and-treat initiative that was rolled out in 2016 will lead to even earlier ART initiation.27
Our analysis has several major strengths. First, we used nationally representative data on CVD risk factors, HIV prevalence and ART coverage to quantify the proportion of individuals with specific risk factors and each individual’s 10-year risk for CVD. We also took into account the effect of HIV and ART on CVD risk factors in sub-Saharan Africa by updating findings of a recent meta-analysis.9 Additionally, our analysis incorporated uncertainty in several parameters by accounting for their distributions and variations in the population. We used the Globorisk CVD prediction model as it could be easily calibrated to fit Uganda’s CVD risk profile,18 to prevent over- or under-estimation of CVD risk when models developed for high-income countries are used in sub-Saharan African populations.28 We also revised the model to include the direct effect of HIV.
Nevertheless, our results should be interpreted with caution. First, as is conventional with most risk prediction scores, our risk equations did not consider competing risks. Although CVD has been shown to be a leading non-AIDS cause of death in PLWH in sub-Saharan Africa,5 there are other common causes of death like tuberculosis. Our risk prediction also did not include non-atherosclerotic CVD events like heart failure and sudden cardiac death that have been linked to HIV-infection.29,30 Second, our model did not incorporate the effects of low immunity and those of some antiretroviral drugs that have been linked to higher risk for CVD.8 Our analysis did not use age-disaggregated estimates of ART coverage and did not incorporate uncertainty in ART coverage because such data were not available. Lastly, as with other models, we are unable to validate the predicted CVD cases due to lack of hard clinical endpoints in this setting.
Conclusions
We found that the predicted burden of atherosclerotic CVD among PLWH remains low despite a high prevalence of raised blood pressure, and that the CVD burden is much higher among HIV-uninfected individuals. HIV treatment programs should prioritize screening and treatment of raised blood pressure so to avoid the missed opportunities that have been previously reported.31 Specific emphasis should be put on studying the clinical effects of Dolutegravir-based ART as countries in sub-Saharan Africa transition to this recommended first line therapy. Addressing the larger burden of CVD and other emerging NCDs among HIV-uninfected individuals will necessitate more empirical evidence on how to build onto existing HIV care and treatment platforms to deliver this care to the general population.32
Acknowledgements: We gratefully acknowledge helpful comments from Kathryn Andrews and Katrina Ortblad on modeling risk factor levels by HIV status and those from Till Bärnighausen, Wafaie Fawzi, Shahin Lockman and Barnabas Nahwera on interpretation of the study’s findings.
Funding: This study was supported by the US National Institutes of Health (NIH) Fogarty International Center through a grant from CRDF Global (Grant # OISE-17-62965-1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health or CRDF Global.
Authorship contributions: SV conceived the study. DG, GM, SB, SO, WM, and PK collected and provided data and advice for the analysis. AK built the simulation model, analyzed data, prepared results and wrote the first draft of the manuscript, under the supervision of SV and GD. NAM reviewed and provided advice on the simulation methods. All authors contributed to writing and reviewing the manuscript. AK and SV had final responsibility for submitting to publication.
Competing interests: The authors completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author), and declare no conflicts of interest.
Correspondence to:
Stéphane Verguet
Department of Global Health and Population
Harvard T.H. Chan School of Public Health
665 Huntington Avenue
Boston MA 02115 USA.
[email protected]

_in_uganda_unde.png)
_cases_in_uganda_from_2016_to_20.png)


_in_uganda_unde.png)
_cases_in_uganda_from_2016_to_20.png)