INTRODUCTION
Over the past two decades, substantial declines in maternal, newborn and child mortality have been observed among least developed countries.1–3 However, preventable deaths take place far too commonly4 with maternal deaths underpinned by pregnancy-induced hypertension, post-partum bleeding and infection5; newborn deaths linked to preterm birth complications, intrapartum events, and infection (sepsis / meningitis)6; and child mortality led by pneumonia and diarrhea incidence.1,6 Despite improved access to healthcare,5 the quality of these services often remains sub-standard.7–10
Systems to ensure availability and access to safe, effective and affordable reproductive, maternal, newborn and child health (RMNCH) commodities can make major inroads in the prevention and management of the most common causes of mortality.5 The UN Commission on Life-saving Commodities for Women and Children (UNCoLSC) was established to articulate key bottlenecks and define strategies to address them. In its report from September 2012,11 the Commission prioritized 13 low-cost and high-impact commodities across the RMNCH spectrum (Figure 1) that if implemented at-scale could make a substantial impact in reducing preventable deaths. The UNCoLSC also outlined a set of recommendations for addressing wider health system challenges that required concurrent attention – from procurement and regulatory systems and supply chains, to up-to-date health worker training materials (Figure 1). While limited in number, the 13 prioritized commodities were intended to act as tracers to provide a high-impact focus in the face of multiple competing priorities.
To take forward the UNCoLSC agenda, a multi-UN agency technical support and financing mechanism was established to accelerate progress towards maternal and child mortality goals among countries where progress was off-track. Three main strategies were implemented.12 First, a country engagement process was undertaken to generate RMNCH acceleration plans. This included a systematic assessment of RMNCH commodity-related bottlenecks, an identification of prioritized interventions, an assessment of technical and financial contributions from development partners (i.e. resource mapping), and an articulation of critical resource gaps. This country engagement process was led by the government, shaped by International Health Partnership principles,13 and informed by global and country-level partners. Second, catalytic financial resources were made available from a multi-UN agency financing mechanism (RMNCH Fund) to support RMNCH acceleration plans. Third, a network of technical resource teams provided additional support to address global barriers and facilitate country implementation.
The aim of this paper is to examine country-level progress against the UNCoLSC recommendations over a five-year period. The specific objectives are to profile availability and access to the 13 commodities and the status of key health systems enablers among high-burden countries; to document specific areas of progress and remaining bottlenecks; and to generate learning to inform the Sustainable Development Goal framework and Universal Health Coverage (UHC) agendas.
METHODS
To assess the baseline status and progress towards the UNCoLSC recommendations, an RMNCH situation analysis was conducted in countries receiving support from the RMNCH Fund. This analysis included a synthesis of existing data sources including governmental and partner documents (e.g. national strategic plans, essential medicines lists, treatment guidelines, regulatory and policy briefs, commodity registers, training curricula), and aggregated quantitative measures from various sources (e.g. health facility assessments, health and logistics management information systems). These data sources were complemented by semi-structured interviews with government officials, programme managers, regulatory and supply chain agencies and in-country partners. The situation analysis and performance indicators (Tables 1 and 2) were designed in consultation with the UNCoLSC technical resource teams (TRTs), a network of approximately 450 experts across 85 organizations, as well as the UNCoLSC Monitoring and Evaluation Advisory Group.12
Within each country, the situation analysis was typically completed over a 2-4 weeks by a trained facilitator, who collaborated with local ministries, UN country teams and in-country partners. These efforts were supported by a multi-UN agency Strategy and Coordination Team (SCT), which facilitated engagement with in-country partners, trained enumerators, coordinated content review, and performed standardized analysis to provide consistency of results across countries and over time. The SCT conducted quality assurance on each country assessment and reviewed the results, data sources, and list of experts interviewed with country teams to ensure accuracy and completeness. While national ministries had discretion over the timing of data collection, this study includes countries where a baseline round of data collection was conducted within one year of program initiation (2014-2015; see Online Supplementary Document Table A) and where a repeat round took place during 2016 or 2017. Results from country assessments were entered into a relational database (MS Access, Microsoft Inc, Seattle, WA, USA), processed using R software (R version 3.2.0, Vienna, Austria), and uploaded to a web-based platform and documented in summary reports for review by country teams. Detailed methods and initial results were previously published.12 All statistical analyses with estimates were conducted in STATA software version 14 (College Station, TX, USA).
Quantitative indicators
Quantitative indicators derived from nationally-representative population- and facility-based surveys are defined in Table 1. To assess change-over-time, indicators from the most recent available survey (i.e. endline) were compared to an earlier data collection (defined as since 2010 and prior to RMNCH Fund initial implementation within each respective country). Selection of data pairs (baseline and endline data points) prioritized comparable indicator generation methods and recency of collection. If data prior to RMNCH Fund implementation was unavailable, then the earliest data source after implementation was used to illustrate trends. In order to identify all available population- and facility-based survey data sources, the SCT consulted with country teams, global UN agencies and development partners to ensure dataset completeness. Results from population- and facility-based surveys were included if available by June 2019. For quantitative indicators, non-parametric paired t-tests were used, due to skewness, to assess statistical significance between baseline and endline distributions.
Categorical indicators
A range of categorical indicators for assessing well-known14,15 in-country health system and commodity-specific bottlenecks were defined through consultation with a global network of technical experts12 (Table 2). A standardized assessment criteria was developed (see Online Supplementary Document Table H) and dichotomous conditions to meet the minimum performance threshold were defined in Table 2 to ensure comparability across countries. These categorical indicators were collected during the country-specific situation analysis with baseline and endline time periods listed in Online Supplementary Document Table A. Indicators related to health systems are reported once per country, while commodity-specific indicators are reported once per commodity per country (i.e. up to 13 times per country). To evaluate statistical significant for categorical indicators, paired t-tests were used between average baseline and endline values.
RESULTS
Between January 2013 to December 2017, 14 countries in sub-Saharan Africa and Southeast Asia underwent two assessment rounds including Bangladesh, Benin, Burkina Faso, Cameroon, Democratic Republic of the Congo (DRC), Ethiopia, Malawi, Mali, Nigeria, Pakistan, Senegal, Sierra Leone, Tanzania and Zambia (see Online Supplementary Document Table A for complete list and timeline of data sources).
Commodity availability and logistics management
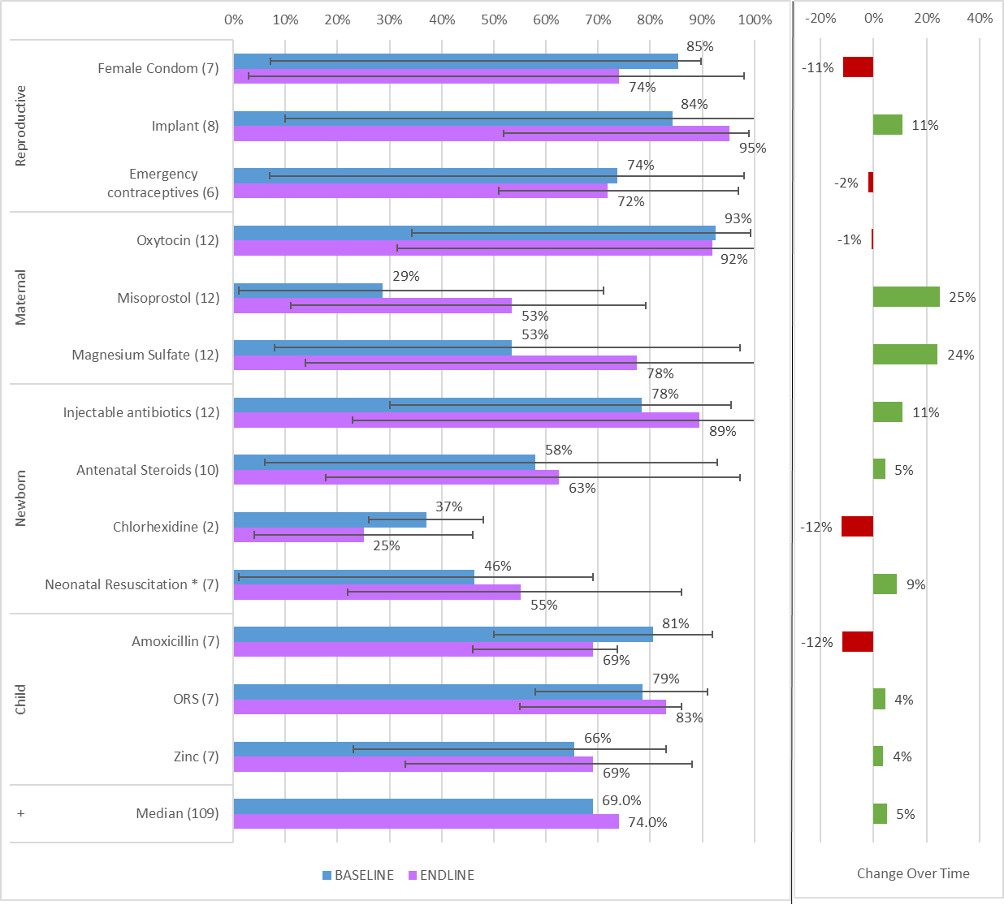
Analysis of stock availability – defined as commodity physically available at health facility at time of assessment - is profiled in Table 1 and disaggregated by commodity in Figure 2. Collectively, there was a statistically significant increase of five percentage points in stock availability of life-saving commodities over the observation period (69% to 74%, P=.001, Table 1). Commodities with the largest improvement included Magnesium Sulfate (24 percentage point increase), Misoprostol (+25 pp) and injectable antibiotics for newborns (+11 pp). Notably, Misoprostol and Magnesium Sulfate had among the lowest baseline availability and thus greatest room for improvement. At most recent assessment, injectable antibiotics, oxytocin and implantable contraceptives were the most widely available (Figure 2). Conversely, a number of reproductive and child health commodities had declined in availability including female condoms (-11 pp), emergency contraceptives (-2 pp), and amoxicillin (-12 pp). In nearly half of facilities, Misoprostol, Chlorhexidine, and neonatal resuscitation equipment were unavailable at the most recent facility assessment.
Stock-outs at national warehouses occurred more often over time (Table 2) and were most prevalent at the most recent assessment for magnesium sulfate, chlorhexidine, and zinc (see Online Supplementary Document Table B). Conversely, female condom, contraceptive implants, and injectable antibiotics for newborns had the fewest national stock-outs by 2017. Across countries, only a handful of commodities (7%) were added into eLMIS to track distribution from central warehouses to service delivery points (Table 2). By 2017, just over half (57%) of life-saving commodity were tracked in eLMIS across countries (Table 2), while comprehensive monitoring platforms exist in less than half of countries.
Health worker performance
Over the observation period, health facilities with a recently trained staff member rose by eight percentage points (P=.003) to 46% (Table 1). In addition, availability of job-aids and/or checklists at health facilities increased to 66% (+13 pp). Moreover, to support workforce performance, 12% and 19% of life-saving commodities were added to national training curricula and job aids, respectively, across countries (Table 2).
Regulatory efficiency and quality strengthening
Regulatory efficiency indicators were the strongest performers over time (Table 2). For example, across countries, an additional 20% of the life-saving commodities were collectively added to national essential medicines lists in the preferred formulation, 11% of commodities were incorporated into national treatment guidelines, and another 12% of commodities were fully registered in-country (each P<.005). Collectively, regulatory indicators also had the fewest outstanding gaps by 2017.
In this study, countries strengthened the sampling and quality testing of drugs (i.e. post-market surveillance) and nearly all countries procured drugs from good manufacturing practice (GMP) accredited manufacturers (Table 2). Unfortunately, the capacity of national control laboratories has declined over time and patient safety monitoring remains low.
Generating Demand and Reaching Women and Children
In this study, modest gains were made in establishing national demand generation and behavior change plans for life-saving commodities that included domestic budget allocations (Table 2). During the observation period, small improvements were seen for removing financial access barriers (i.e. user fees); however, gaps still exist in approximately half of service delivery areas across countries (Table 2). Where data were available, coverage rates (i.e. percent of affected population receiving appropriate treatment) increased two-fold over the observation period (P=.111), but remains low for many commodities (Table 1), such as ORS (median 37%), Zinc (27%), and contraceptive implants (6%) (Online Supplementary Document Figure F).
Other Health System Enablers
Over time, results-based financing programs were more prevalent and all 14 countries had RMNCH coordinating mechanisms by 2017 (Table 2). However, countries struggled to finalize national costed RMNCH plans with secured domestic budget allocation as well as develop commodity security strategies.
DISCUSSION
This study assessed the ambitious UNCoLSC recommendations to improve availability and access to 13 life-saving commodities among countries in Africa and Asia with among the highest global burden of preventable maternal child deaths. Service readiness, including availability of commodities, improves the likelihood of receiving good quality services.7,16–18 Over the observation period, commodities were collectively more available; however, some commodities fared better than others (Figure 2). Commodities to reduce maternal deaths, treat newborn infection, and long acting methods for pregnancy prevention became more widely available. Scale-up of contraceptive implants have been a concerted focus of recent international and domestic efforts,19,20 while the low cost and long regulatory history of oxytocin and injectable antibiotics, such as gentamicin, facilitate efficient delivery through the supply chain.21–23 Conversely, access to misoprostol, chlorhexidine, antenatal corticosteroids and newborn resuscitation equipment still hovers around half of health facilities. Emerging science on the effective use of Chlorhexidine24 and antenatal corticosteroids15,25 produced operational re-examination, clouded guidance and limited scale-up. While the administration of misoprostol to prevent and treat post-partum hemorrhage is expanding to home-births,26 it can also be used to induce abortion, which can limit cultural adoption and the need for local stock availability.
Maintenance of national stock levels for essential commodities declined over the observation period and requires urgent attention. Increasing the use of centralized procurement through partners, such as family planning commodities, is cost-effective and efficient, but needs complementary in-country coordination.27,28 Often the discordance of national budget and cash flow cycles impedes the efficient bulk purchase of commodities by national governments. Innovative bridge funding mechanisms or working capital facilities (with prompt payback terms) to support local procurement through established systems could reduce stock-outs and save money.29 Once procured, ensuring efficient, timely and equitable distribution of commodities within country relies on electronic logistics management information systems (eLMIS). In-country supply chains are often fragmented across multiple partners along with the corresponding eLMIS, which limits performance and timely corrective action.30,31 The Health Data Collaborative (HDC) has set out to support the harmonization and more efficient use of information systems.32 Expanding the domain of HDC or applying the same principles to complex issues such as supply chains and eLMIS is warranted to reduce fragmentation and improve efficient data use.
Inadequate workforce competences is an underlying operational barrier to effective healthcare delivery.14,33 To further strengthen the patient-provider interface, countries in this study improved national health workforce resources (e.g. training curricula and job-aids / checklist) as well as facility-based training. Since countries trained staff at just under half of health facilities within 12 or 24 months, the ability to reach all facilities could take approximately three to five years (assuming equitable distribution). This collective training capacity should inform introduction rates for new commodity guidelines and technology in the future. Prioritized investments and improved metrics on health workforce training and guidance (e.g. job-aids) portend enhanced quality of service delivery. A new opportunity for acceleration is the Network for Improving Quality of Care (QOC) for Maternal, Newborn and Child Health,34,35 which aims to establish local quality improvement teams to identify quality shortfalls and undertake quick cycles of problem-solving for facility-driven improvement and preparedness. Unfortunately, the utilization and quality of facility-level care is clouded by the lack of coverage data36–38 for administration of life-saving interventions at health facilities (e.g. oxytocin, injectable antibiotics). Moreover, while this study had access to the health management information systems, such as DHIS2, in most countries, the data available from these sources at time of assessment was deemed insufficient to generate a valid estimate of intervention coverage over time.
Delays in regulatory approval limit pharmaceutical companies interest in providing drugs to developing countries, which creates a market opportunity for an influx of counterfeit drugs.39,40 In this study, regulatory indicators exhibited significant improvement; however, they require regular review to ensure the latest treatment guidelines and formulations are adopted and product registration does not expire. With the rise of counterfeit drugs in circulation,41 countries need quality controls and monitoring of medicines to ensure the safety and effectiveness of drugs is intact when reaching women and children. Aside from post-market surveillance, there was little to no improvement in quality strengthening conditions, which have been persistent intractable bottlenecks.39 Several interconnected regional efforts, such as the African Medicines Regulatory Harmonization (AMRH) Initiative,42 continue to strengthen fragmented regulatory environments and improve the availability of safe and effective commodities.
The collective improvements against the UNCoLSC recommendations mirrored the predominance of supply side investments prioritized by country teams and financed via the RMNCH Fund (see Online Supplementary Document Figure B). More than 80% of these expenditures were allocated towards two UNCoLSC recommendations: supply and awareness (e.g. commodity procurement, integrated LMIS, and supply chain management); and health worker performance and accountability (e.g. staffing, training, supervision, job-aids / checklists). These RMNCH acceleration plans were developed by government-led RMNCH coordinating mechanisms using a resource mapping across partners43,44; therefore, expenditures against the RMNCH Fund provide a window into national priorities and funding gaps facing governments, UN agencies and development partners (see Online Supplementary Document Table G). An example is provided below (Box 1), which outlines the experience of the United Republic of Tanzania with the RMNCH Fund. In terms of sustainability, the RMNCH Fund provided short-term catalytic funding towards the UNCoLSC agenda. This short-term funding is small relative to potential domestic allocations; however, domestic budget allocations were often unpredictable and vacillated year-to-year across countries (Table 2). This type of shorter-term gap filling to support existing country-derived strategic plans should complement new longer-term funding sources, such as the Global Financing Facility, as they are actuated and put towards the same strategic vision.45,46
There were several limitations in this analysis that are important to draw attention to. First, gaps in data availability, such as maternal and newborn service delivery at health facilities, persist and constrained interpretation of community impacts. This study used the RMNCH Situation Analysis process, which is relatively easy and low-cost to conduct, but relies on existing data sources.44,45 The tool can be adapted for country context, such as sub-national use or expanded commodity and equipment list, but d.oes not establish new primary quantitative datasets for analysis. Second, the identification of operational bottlenecks relies on performance thresholds defined by a panel of experts; however, during enumeration, participant input and categorization by individual enumerators may be subjective and introduce bias. Third, countries assessed that met the requirements for this study (e.g. multiple data collection rounds of the RMNCH Situation Analysis) may not represent conditions experienced in other countries or regions. Fourth, this study did not analyze global-level UNCoLSC recommendations (e.g. product innovation and market shaping), which were assessed in a previous study,12 but could affect progress against related in-country bottlenecks. Next, this study utilized point estimates from facility- and population-based surveys to statistically analyze trends. The raw datasets had limited availability and their analysis was beyond the scope of this study, but may generate more decisive results and worthy of future research. Finally, the timeliness and consistency of available data sources may obfuscate recent changes. For example, this study utilized health facility assessments supported by various international institutions, including UNFPA (service delivery point survey), WHO (service availability and readiness assessment), and USAID (service provision assessment). While UNFPA has by far the most frequent data collection, the WHO and USAID tools are more comprehensive. Unfortunately, the tools are not entirely compatible among agencies (such as content and methodologies) and collection schedules are uncoordinated. The inter-agency HDC has undertaken an effort to harmonize these tools and data collection schedule among partners.32
CONCLUSION
Over a five-year period, significant improvements in commodity availability and health workforce training at the facility level were observed overall. Important commodity-related bottlenecks, such as coordination mechanisms, regulatory requirements, and national training curricula and job-aids, were near fully rectified. However, critical supply chain and medicine safety functions showed inconsistent improvement and remain an impediment to universal access. Leveraging the lessons learned from this unfinished UNCoLSC agenda can help in-country and global initiatives – such as the African Medicines Regulatory Harmonization, Global Financing Facility, Health Data Collaborative, and Quality of Care Network - address these remaining barriers to women and children receiving life-saving commodities and ultimately reaching universal health coverage.
Acknowledgements: Special thanks to Desmond Koroma, Lazasoa
Raharimanjato, Kavitha Viswanathan and Amani Siyam.
Funding: No funding was received for the writing of this manuscript. [Note: Financing for RMNCH Fund was provided by Governments of Norway (NORAD) and United Kingdom (DFID), but they had no role in the writing of the manuscript.]
Author contributions: BN, DS, NS, and PP drafted the manuscript. All authors contributed to the design of the assessment, analysis of data, writing of the manuscript, and agree with the results and conclusions.
Competing interests: The authors completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author), and declare no conflicts of interest.
Correspondence to:
Bennett Nemser, MBA, MPH
University of the Western Cape,
Cape Town, South Africa
[email protected] or [email protected]