Obesity is a global epidemic. The World Health Organisation reported that obesity worldwide has tripled since 1975.1 In 2016, over 650 million adults (>18 years) were obese, and 41 million children (age 5-19) were overweight or obese.1 The Organisation for Economic Co-operation and Development countries 2017 Obesity Update ranked United States of America as the first and NZ as the third most obese nations in 2015.2 The 2018/2019 New Zealand Health Survey reported 31% adults (>15 years) and 11.3% children (2-14 years) were obese with highest rates among Pacific adults (Pacific is an ethnic or racial term to explain people of the Pacific Islands) and Māori (Indigenous people of New Zealand) compared with European/Other and Asian (66.5%, 48.2%, 29.1%, 13.8%, respectively).
Counties Manukau Health (CMH) in South Auckland, NZ is an area of high deprivation and home to multi-ethnic populations with high rates of obesity in pregnancy, with rates in Pacific, Māori, European, Indian, Chinese and Asian/Other of 65%, 45%, 26%, 13%, 4% and 6%, respectively.3 Obesity during pregnancy is a risk factor for most pregnancy complications including gestational diabetes, pre-eclampsia, caesareans, difficult or assisted births; and in the offspring congenital abnormalities, stillbirth, foetal overgrowth and preterm births,4 with negative impact on long term health of the offspring.5 Excessive gestational weight gain (GWG) during pregnancy also increases pregnancy complications, and the long-term health consequences are similar to those that occur with obesity.6
A recent study identified that >70% of CMH women booked for antenatal care gained excess weight in pregnancy.7 Obesity is not modifiable during pregnancy but limiting weight gain may improve pregnancy outcomes.8 Women with obesity tend to gain excess weight during pregnancy.6 A review of CMH maternity care in 2012 highlighted the association between obesity and high perinatal mortality, especially in Pacific women and recommended that culturally appropriate interventions should be developed to limit pregnancy weight gain.9
The recently published Healthy Mums and Babies (HUMBA) randomised controlled trial (RCT) was carried out in a multi-ethnic high deprivation cohort of pregnant women with obesity residing in the CMH region.10 In HUMBA, we assessed whether a culturally tailored dietary intervention versus routine dietary advice, with or without probiotics could reduce maternal excessive weight gain and optimise infant birthweight. The published HUMBA trial demonstrated that women who received the dietary intervention had less total pregnancy weight gain of -1.76 kg (95% confidence interval CI -3.55, 0.03), but there were no significant differences in the primary outcomes (proportion of women with excessive pregnancy weight gain or birthweight) and there were no health benefits from probiotic treatment.10
This evaluation was conducted due to the scarcity of information from multi-ethnic populations about participation in research and concern that low socio-economic multi-ethnic populations (Māori and Pacific) with poorer health outcomes are less likely to participate in research that may improve their health.11 A systematic review in minority populations in the USA (Pacific Islanders included) identified shared barriers of mistrust, concerns about payment, not understanding research, historic stigma, uncertainties about adverse effects and language barriers.12 Due to limited information of how multi-ethnic populations felt about participating in research, we sought feedback from participants in the HUMBA trial specifically on their experiences in taking probiotic or placebo capsules; experiences with the oral glucose tolerance test (OGTT); and in the dietary intervention subgroup, feedback about CHW contribution to dietary changes, and their views about text messaging. We investigated the relationship between high satisfaction and weight-gain and birthweight; and the relationship between satisfaction and maternal characteristics.
METHODS
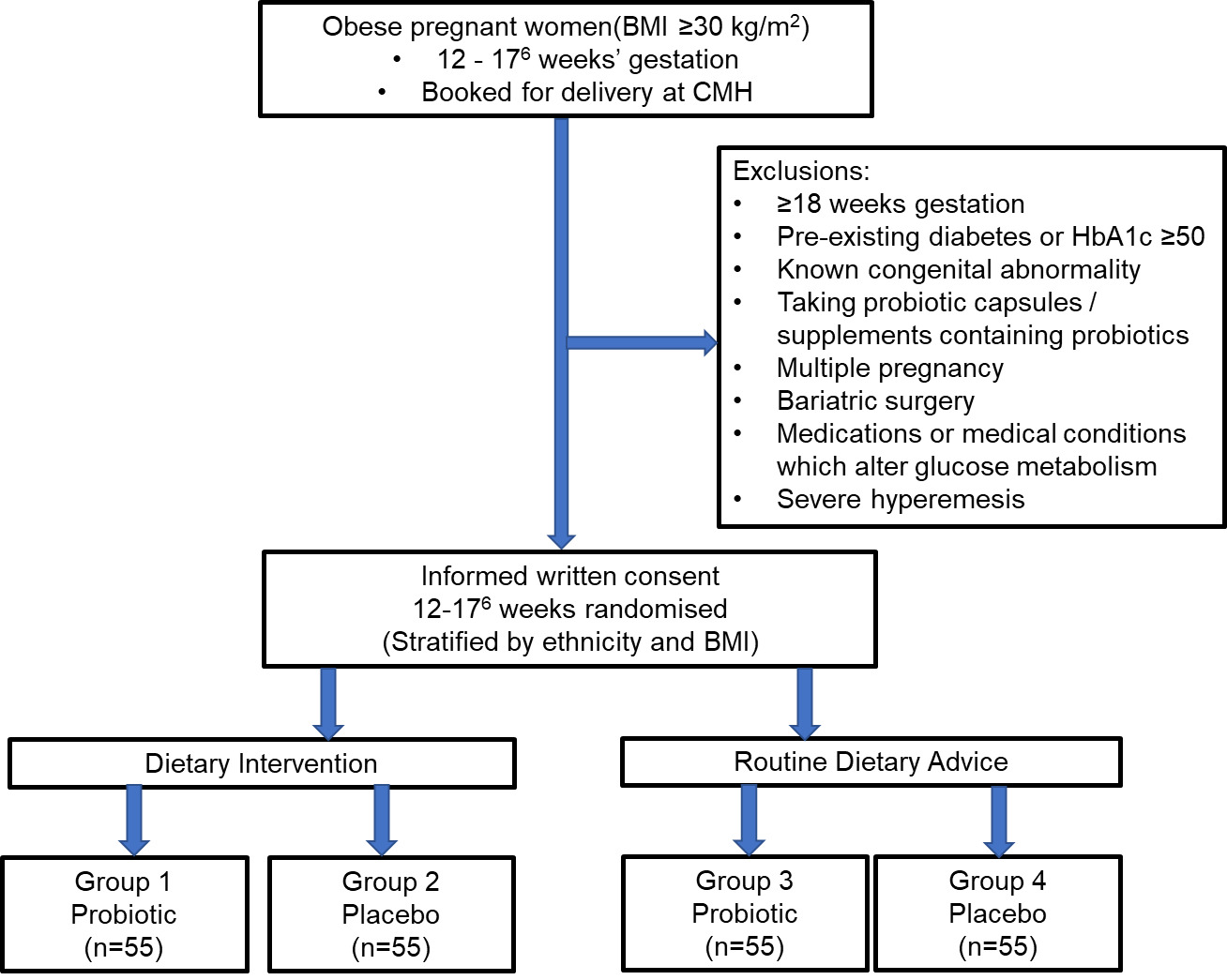
Detailed methods for the trial are described in the HUMBA protocol.13 Study processes are summarised in Figure 1. This was a single centre randomised controlled trial in the CMH Region in South Auckland, NZ. The trial was registered with the Australian New Zealand Clinical Trials registry (ACTRN12615000400561). Ethics approval was obtained from the Southern Health and Disability Ethics Committee, New Zealand (14/STH/205).
Women randomised to dietary intervention received four visits by a CHW who had completed the Certificate of Proficiency in Pacific Nutrition (Auckland University of Technology) and the Healthy Conversations training by Gravida.14 CHWs also received education about the effective delivery of healthy eating advice in pregnancy from an experienced clinical dietitian. The dietary group received a handbook with information about unhealthy drinks; healthy food varieties; portion sizes; healthy recipes; reading food labels; takeaways; managing cravings; how to set SMARTER (Specific, Measurable, Action, Realistic, Timed, Evaluated, Reviewed) goals and ways to be more physically active. The handbook contained a weight gain chart for the woman to plot and monitor her weight gain during visits and throughout pregnancy. CHW’s had specific topics to discuss and developed goals with each woman. The four visits were to be completed before the 26-28 weeks oral glucose tolerance test (OGTT). This group also received supportive text messages about healthy eating and physical activity until birth. Women in the routine dietary advice arm received a best practice written dietary information booklet from the NZ Ministry of Health titled “Healthy eating and healthy weight gain in pregnancy”.15 Healthy advice in this booklet included information about food varieties and serving sizes; drinking more water or low-fat milk; preparing food low in fat, salt and sugar; food safety; pregnancy supplements and how to cope with pregnancy symptoms. Probiotics (Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12 at 6.5 x109 colony forming units) and placebo (identical capsules containing microcrystalline cellulose and dextrose anhydrate) capsules were allotted at recruitment and continued until birth.
At the time of our questionnaire development, there were no suitable validated questionnaires available. With assistance from an academic with expertise in questionnaire development and analysis (MH) and the dietitian who provided oversight for CHWs for the dietary intervention (DN), we developed a succinct, easily understandable questionnaire suitable for a multi-ethnic sample of NZ women. A six-point Likert scale was utilised beginning with 1=never agree to 6=always agree. The questionnaire was trialled amongst community midwives and the research team (CHWs, obstetricians, midwives) to check suitability and ease of administration. Participants in HUMBA completed the questionnaire within 72 hours of birth.
Data and statistical analysis
We assessed the internal consistency of the questionnaire sections using Cronbach’s alpha, with a high internal consistency coefficient indicated by coefficient α >0.70.16 These coefficients were appraised for each of the four sections: 1) Overall Experience, 2) Capsules, 3) CHWs and 4) Text messaging. Questions in section 3 were summed to create a “Community Health Worker Satisfaction” summary score and questions in section 4 were summed to create a “Text Messaging Satisfaction” summary score. Data were analysed using the Statistical Analysis Systems (SAS v 9.4, SAS Institute., Cary, NC, USA) to explore the relationships between participant satisfaction and the primary outcomes of excessive GWG and baby birthweight using binary logistic regression and linear regression, respectively. The relationship between “Text Messaging Satisfaction” and maternal age was also assessed using linear regression, and the relationship between “Community Health Worker Satisfaction” and ethnicity was assessed using analysis of variance (ANOVA).
Qualitative content analysis
The qualitative content analysis17 was conducted on the responses from the six pre-specified questions about participants’ experience with the OGTT, difficulties with being fully involved in HUMBA, ease with taking the HUMBA capsules, partner support and recommending or not recommending the HUMBA capsules to a friend.
RESULTS
Quantitative
A total of 230 women were recruited to the HUMBA trial.10 Participant demographics were balanced across groups (dietary intervention, routine dietary advice, probiotics and placebo). The mean overall age of participants was 29 years; gestation at recruitment 15 weeks; and booking BMI of 38.6 kg/m2. The ethnic distribution in the sample was broadly representative of the birthing population in CMH: NZ Māori (Indigenous people of New Zealand) 22%, Cook Island Māori (Indigenous people of Cook Islands) 12%, Samoan (Indigenous people of Samoa) 20%, Tongan (Indigenous people of Tonga) 12%, Other Pacific 6%, European 18%, Indian 6%, Other 4.0%. About 30% of participants did not complete high school, 50% were in paid employment, 64% resided in the highest deprivation quintile for NZ; 54% were married or in a civil union, and 14% were current smokers. Over 80% completed the post-natal questionnaire (dietary intervention, N=98 [84%], and routine advice, N=97 [85%]).
Participants (N=194) reported that their overall experience was positive (92%); it was easy to take the capsules (80%), felt their partner was supportive of them taking the capsules (89%); and if the capsules improved the health of mothers and babies, they would recommend them to a friend (97%). Two-thirds (67%) considered that the OGTT was easy to complete (Table 1).
Participants in the group randomised to the dietary intervention implemented by the CHWs, reported that in between visits they made some changes to their food intake (70%); the education helped them to eat healthy (73%); the goals set together with the CHW helped them change their diet (62%) and to try to keep to the recommended weight gain (63%). Most would recommend the CHW intervention to a friend (82%) (Table 2). Furthermore, participants in the dietary intervention reported that they read their text messages (93%); it helped them to eat healthy (65%), receiving text messages from the baby was a good idea (79%), and they would recommend HUMBA text messages to a friend to assist with healthy eating (83%) (Table 2).
With regards to physical activity, participants in the dietary intervention arm reported that: between HUMBA visits from the CHW they made changes to their physical activity (41%); the education received helped them be more physically active (40%); goals set with the CHW increased physical activity (37%); and HUMBA text messages helped them become more physically active (46%).
In the four sections of the postnatal questionnaire the alpha coefficients were: i) overall experience, α =.10, ii) capsules, α =.50, iii) CHW, α =.88 and iv) text messaging, α =.84, the latter two variables thus being potentially useful for further comparative analysis. The internal consistency of the variable CHW was improved (α =.90) by removing the question “number of visits with CHW was just right”. The internal consistency of the variable, text messaging was improved (α =.89) following removal of two items, namely “I read my HUMBA text messages” and “The HUMBA text messages helped me to be more physically active”.
Composite measures were computed for these revised variable versions. In reference to the CHW variable, the results from the ANOVA indicated that satisfaction regarding CHWs did not differ by ethnic group (P=0.25). In the regression analysis, there was no significant relationship between CHW satisfaction score and excessive GWG (odds ratio OR=1.00, 95% confidence interval, CI=0.95 to 1.05), or birthweight estimate (-3.96 g, 95% CI= -15.89 to 7.98).
In reference to the ‘text messaging’ variable, the binary regression analysis indicated no significant relationships between text messaging satisfaction and excessive GWG (OR=1.06, 95% CI=0.94 to 1.19), or birthweight estimate (-8.52 g, 95% CI= -38.67 to 21.64). Furthermore, there was no significant relationship between text messaging satisfaction and maternal age (P=0.73).
Qualitative
As shown in Table 3, the qualitative analysis indicates four key categories: i) satisfaction with HUMBA involvement, ii) ease with taking capsules, iii) recommending HUMBA capsules, and iv) reasons for not using HUMBA messages. Feedback for the OGTT was also collected.
i) The positives of being involved in the HUMBA trial were around enjoyment, feeling good, increased motivation, helpful communication with researchers, and meeting times organized at a suitable time. The challenges were around full homes, busy lives, and shift work.
ii) With the HUMBA capsules, of those who responded, 35/45 (78%) reported issues with side-effects, non-compliance, forgetting pills and reduced motivation. Very few reported no issues with taking pills; and good partner support.
iii) Participants would recommend HUMBA capsules to a friend if it improved the health of mother and baby.
iv) HUMBA messages would not be read if the mother: felt they were irrelevant; knew she was already eating healthy and exercising during pregnancy; and considered them almost annoying.
Women who provided feedback for the OGTT, 56/86 (65%) reported difficulties with or declined the test. Main issues were unpleasant taste, side effects; time taken and personal costs. One woman reported dislike of extra tests due to lab error.
DISCUSSION
This RCT of nutritional interventions in pregnancy recruited predominantly Māori and Pacific women with a high BMI where most resided in an area of high deprivation.
The finding of this evaluation provided information regarding participant experience whilst involved in the HUMBA RCT. The findings can be considered in terms of i) Overall experience and factors that may have assisted with participation, ii) Benefits of Community Health Worker, iii) Use of text messaging systems, iv) Probiotic or placebo (HUMBA) capsules, and v) Oral glucose tolerance test (OGTT).
Overall experience and factors that may have assisted with participation
Most women reported that their overall experience of participation in the HUMBA trial was positive, regardless of the intervention they received. A minority reported having difficulties being involved in HUMBA. Those who reported positively, found the knowledge helpful and that attending appointments was easy as they were organised around their daily timetable.
Although the limited existing literature identified high risk populations (such as Māori and Pacific) as suspicious and frequently declining participation in research,12 we were heartened by the willingness of women from all ethnic groups residing in this region of predominantly high deprivation, to participate in HUMBA. Pacific researchers have raised concerns about Pacific populations being over researched due to poor outcomes, with research perceived as being invasive and leading to research suspicion, fatigue, and lack of interest.18 Other concerns were around collecting information from Pacific communities and data misinterpretation by researchers due to cultural misunderstanding. Similar issues were also experienced by Māori with the result that Lawton and her Māori researcher colleagues19 developed a kaupapa Māori research framework to help reduce health disparities in young Māori pregnant mothers. Prior to initiating HUMBA, we undertook community consultation by means of focus groups which contributed to developing a survey in a cohort of multi-ethnic women in late pregnancy from the region. The majority of survey participants responded that they would participate in a trial of dietary interventions in pregnancy if it had potential to improve their health and the health of their baby.20 This pre-HUMBA consultation and community feedback may have facilitated the success of participation in HUMBA. Facilitators for successful research have been reported as having researchers with a good cultural understanding of the population studied and if their research may have a high chance of improving the health of future generations.12
Employing researchers reflective of the ethnic background of the community may also improve participation in research.21 The multi-ethnic composition of the research team, which included Māori and Pacific researchers (Māori and Pacific CHW providing the intervention and a Pacific obstetrician), an Asian coordinator, and European researchers with a good understanding of the population, may have assisted with successful recruitment and retention in HUMBA. Carers for pregnant women in the region had HUMBA fully explained and were encouraged to promote the study and refer eligible women. The trusting relationship between maternity carers and the women,22 may have assisted recruitment and retention similar to women in a breech study who reported they were more likely to participate if they were encouraged by their carer, given adequate information, and personal altruism.23
Qualitative evaluation of women’s feelings about participating in a randomised trial identified the main motive as contribution to scientific research. Non-participation in research has been reported as due to dislike of an intervention, concern of the possibility of harm, and practical reasons.24
Benefits of community health worker
Women randomised to the dietary intervention, reported that contact with a CHW was a very positive experience. Cronbach’s alpha results indicated that participants responded to the items by the CHW’s similarly. In between visits, women reported that they had made changes to their food intake and the advice by CHW helped them to eat healthily. Goals set with the CHW helped them to change their diet and try to keep to recommended weight gain. The majority reported they would recommend the CHW to a friend. However, we were unable to demonstrate a relationship between high CHW satisfaction and our main study outcomes of excessive GWG or birthweight. Our finding of reduction in total weight gain (secondary HUMBA outcome) in women who received the dietary intervention from CHW was encouraging10 if sustained and if it results in long-term health benefits for mums and babies.
A similar pilot of lifestyle intervention with lifestyle goals delivered by CHW for prevention of diabetes in non-pregnant Māori populations in NZ reported that the delivery approach was acceptable to participants. Those diagnosed with impaired fasting or impaired glucose tolerance test, had significant weight loss (5.2 kg [SD 6.6], P <0.01) in the intervention group.25 Community health workers assisting in delivery of diabetes intervention in a non-pregnant adult population in Samoa and Hawaii were effective in lowering HBA1c levels compared to controls mean (SD) (2.2% [1.8], P<0.01).26 We found that a minority of participants in the dietary intervention reported that the advice received helped them to increase physical activity, similar to findings in the large UK UPBEAT trial of nutrition and physical activity in pregnancy.27 CHWs have been recognised as able to bridge the gap between vulnerable communities and healthcare; are natural researchers and much better at understanding their communities and issues affecting the community socio-economically and in health matters.28 CHWs in CMH assist our community midwives to support and encourage vulnerable women to attend appointments and provide advice on safe sleep practices with use of “wahakura” or “Pepi-pod” (Whahakura is a flax bassinet with a small mattress inside as a separate sleeping surface for the infant. Pepi-pod: pepi = baby in Māori and pepi-pod is similar to the whahakura but is made of other materials like plastic). Whahakura and pepi-pods are distributed in NZ to reduce the risks of Sudden death of the infant and encourages a separate protected sleeping space for the baby. CHWs post HUMBA trial have extended their role in assisting our diabetes in pregnancy midwives and women with gestational diabetes (GDM) to do blood capillary meter reading and offer dietary advice. Other centres in NZ with limited resources may wish to trial delivery of care by CHWs, especially in regions with vulnerable high-risk populations.
Text messaging
Text messaging to support the dietary intervention was positively received with most women reading their text messages and the majority reporting the texts helped them to eat healthy. Women enjoyed and approved of receiving text messages from the baby. Again, although there was positive feedback from the women in the descriptive analysis, it was found that there was no significant relationship between text message satisfaction and GWG or birthweight. There was no significant relationship between text messaging satisfaction and maternal age.
There was limited literature detailing pregnant women’s evaluation of participation in a dietary text-messaging intervention during pregnancy. Studies using text messaging interventions for weight loss in non-pregnant participants reported high scores in user satisfaction29 participants would recommend text messaging weight loss interventions to friends and family.30
Probiotic or placebo (HUMBA) capsules
Most women reported it was easy to take the HUMBA capsules, and where applicable, that their partner supported this. Most reported they would recommend the capsules to a friend if probiotics were shown to improve the health of mothers and babies.
Although there have been many trials of probiotics during pregnancy31 apart from most acknowledging their safety,32 there is a scarcity of literature evaluating women’s feedback on participation in these trials. One trial evaluated maternal feedback on use of probiotics in their infants33 reported: majority had heard of probiotics (99%); were aware they contained live bacteria (87%); had used probiotics themselves (89%); and believed they were beneficial (73%). None thought they were harmful, but only 51% gave probiotics to their infants.33 Although women are aware probiotics contain live bacteria, it appears that probiotics are well tolerated in pregnancy and the majority of women have no hesitation in using them in the hope for health benefits. Disappointingly increasing evidence does not support use of probiotics to reduce GDM and other pregnancy complications.34
Oral glucose tolerance test (OGTT):
Surprisingly two-thirds of women reported it was easy for them to do the OGTT. Women who reported difficulties with the OGTT commented mostly around taste, nausea and vomiting, and amount of time required to do the test. The issues expressed by women in HUMBA are also echoed by others in the literature with the test being time consuming, unpleasant causing vomiting and inability to complete the test with estimated failure rate of 10%.35 The predictive value of the OGTT varies with different ethnic groups.36 There is need for a different diagnostic test for GDM during pregnancy but for the time being, we have to continue with the OGTT.
Limitations
As this was a trial involving multiple groups (midwives, dietitians, Pacific Heartbeat and others), we were not able to apply the Pacific research methodology of “talanoa” or “korero” in Māori, a common cultural method of gathering information through story telling.18 Answers to some questions were limited (Table 2) and unable to provide in-depth information. Further questions to identify the types of changes would have provided more information. We were limited in time and being mindful of the woman’s time with a new-born, administering long questionnaires may be unacceptable.
CONCLUSIONS
Carrying out important research in high risk multi-ethnic populations with obesity during pregnancy, in areas of high deprivation is possible and feasible provided it is developed and implemented in a careful and culturally appropriate way. Women enjoyed the contact with research midwives and CHWs. Text messages were viewed positively. Although most were happy to undertake the OGTT, a proportion of women struggled with side effects. Despite positive feedback from participants regarding their contact with CHWs, high satisfaction was not associated with the primary study outcomes.
Acknowledgements: We wish to thank the women who participated in HUMBA. We also wish to thank all the midwives and lead maternity carers who referred women to us to participate in the HUMBA trial. We wish to thank research midwives: Cecile O’Driscoll, Sarah Va’afusuaga, Susan Ross-Heard, Annette Hallaran and paediatric nurse Megan McCowan; the HUMBA Community Health Workers: Eseta Nicholls, Kristine Day, Mele Fakaosilea; Project managers Shireen Chua, and Noleen van Zyl; Dr Rebecca Pullon for statistical assistance. We are grateful to Pacific Heartbeat and the Heart Foundation of NZ for the professional development of community health workers (AUT Certificate of Proficiency in Pacific Nutrition) and assisting with nutritional resources and text messages. We wish to thank Gravidae (Liggin’s Institute) for training CHWs in “Healthy conversations”. We also wish to thank Professor Lucilla Poston for sharing nutritional resources used in the UPBEAT Trial, and Professor Leonie Callaway for sharing the SPRING Trial protocol.
In kind support: Christian Hansen A/S for probiotics and placebo capsules; Roche International Diagnostics Ltd for HBA1 and Lipids bedside testing.
Funding: This study was funded by Cure Kids (NZ), Counties Manukau Health, University of Auckland Faculty Development and Research Fund and Re-investment Fund, RANZCOG Mercia Barnes Trust, Nurture Foundation, Gravida National Centre for Growth and Development and Lottery Health Research. Funding sources had no role in the study design, data collection, analysis, interpretation, report writing or decision to submit this paper for publication.
Competing interests: The author completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author) and declare no conflicts of interest.
Authorship contribution: KOG, LMcC, CM, DN, RT, CW, JT, CC, and MH were involved in the design of this study. KOG drafted the manuscript. LMcC, CM, DN, RT, CW, JT, CC, and MH critically evaluated the manuscript. JT, JW and MH designed and JT and JW carried out the required statistical analysis for this study. All authors have read and approved the final manuscript prior to submission for publication.
Correspondence to:
Dr Karaponi A.M. Okesene-Gafa, FRANZCOG
Department of Obstetrics and Gynaecology
University of Auckland
Private Bag 92019
Auckland 1142
New Zealand
[email protected]