Over 1.6 million Kenyans live with HIV, constituting a 5.4% adult prevalence of HIV. 1 Kenya currently has the fourth-largest HIV epidemic in the world; however, much progress has been done to combat this scourge. Over the last few years, the Kenyan Ministry of Health has aggressively scaled up antiretroviral therapy (ART). Presently, 65% of children living with HIV and 80% of pregnant women living with HIV have been linked to treatment. 1 Similarly, Kenya has also advanced education on methods of prevention, with just under 60% of Kenyans between 15 and 24 years of age having knowledge of HIV prevention techniques. 1 Continued expansion of ART and other prevention methods will continue to decrease the incidence of HIV.
Beyond targeting the HIV epidemic biomedically, the Kenyan government has also established critical social protection programs. With support from the international community and the appropriate political climate, the Kenyan government implemented its first unconditional cash transfer program in 2004, intended to help orphans and vulnerable children (OVC) buffer the stress associated with losing one or both parents to AIDS. 2 Previous research has shown that the OVC cash transfer program is associated with decreased risk of early sexual debut 3 and early pregnancy. 4 However, since 2004, the government has introduced several other cash transfer programs, including elderly, urban food subsidy, and hunger safety net programs. Our research adds to the growing literature on the benefits of Kenyan unconditional cash transfer programs by examining the population-wide associations between receipt of cash transfers and various HIV risk indicators. We include a more comprehensive coverage of the diverse range of cash transfer programs available in Kenya and analyze the most recent nationally representative data available using a rigorous, matching method.
Cash transfer recipients generally come from a lower socioeconomic background, are less educated, and possess lower levels of health literacy. Unconditional cash transfers are intended to provide individuals and households with supplementary income to protect them from the ills of poverty. 5 We hypothesize that as a result of receiving cash transfers — compared to those who do not receive cash transfers — recipients will be less likely to engage in HIV risk behaviors, such as transactional sex and age-disparate sex, and more likely to engage in safe sex practices, such as using condoms and getting tested for HIV. We believe that cash transfers confer these health benefits on their recipients through increased linkage to social services. Because recipients have increased interactions with the Kenyan government as a result of receiving cash transfers, they are likely to be exposed to more health communication, which can increase their own general health literacy. Thus, those who receive cash transfers should have higher levels of knowledge when it comes to HIV prevention, allowing them to reduce their risk of infection.
METHODS
Background on the Demographic and Health Surveys
The Demographic and Health Surveys (DHS) are nationally representative surveys administered by the United States Agency for International Development (USAID) in conjunction with target nations approximately every five years. 6 Incorporating large sample sizes, these surveys employ a stratified two-stage cluster design: clusters are first identified via census data and then individual households are selected from each cluster. 7 These households and the individuals within them respond to questionnaires administered by trained staff members. The survey items are designed to collect data on a variety of health measures, including maternal health; education; HIV/AIDS knowledge, attitudes, and behavior; and wealth. The results from these surveys are used to help inform and shape health policies. We use the latest DHS conducted in Kenya: the 2014 Kenya DHS (KDHS).
The KDHS is a nationally representative survey for Kenya that sampled 36,430 households and collected detailed health and sociodemographic information. A total of 1,612 clusters — 617 urban and 995 rural — were selected from the master frame. These clusters were randomly drawn from enumeration areas used in the 2009 Kenya Population and Housing Census. Because our research question focused on the receipt of cash transfers, HIV risks and knowledge, our final analytic sample consists of 12,800 unique responses from individuals between the ages of 15 and 54. Our analytic sample is representative of the overall sample and mirrors its distribution on all relevant demographic characteristics.
Of note, potential biases stemming from data collection are limited because of the extensive training of interviewers, supervisors, and quality assurance personnel as well as the comprehensive measures put in place to monitor uniformity of data collection and adherence to survey protocol. All collected data were double entered and validated. However, biases could have been present in the form of recall bias and non-response bias. Recall bias is always to be expected in survey-based studies, but the large sample size present should allay concerns of skewed results. Additionally, the overall response rate was 99%, which mitigates major concerns regarding non-response bias.
Exploratory data analysis
All analyses were conducted in Stata version 15.1 (StataCorp LLC, College Station, TX, USA). 8 We first merged the individual, men, and household questionnaires to create a master dataset. We then conducted preliminary analysis of the association between receipt of cash transfers and respondent characteristics using unequal variances t-tests and chi-square tests.
Propensity score matching
Because of our exploratory data analysis, we recognized that those who received cash transfers had different baseline characteristics than those who did not. Thus, we turned to propensity score matching to reduce these observable differences between the treatment and control groups and produce more comparable groups. Propensity score matching estimates the effect of a treatment by accounting for covariates that predict treatment assignment. We utilized Becker and Ichino’s pscore Stata package to perform matching. 9 This algorithm first runs a logistic regression with the treatment variable as the dependent variable and the selected covariates to match on as the independent variables to calculate the propensity score for each individual, which is the conditional probability of receiving a treatment given pretreatment conditions. 10 Next, pscore splits the sample into equally spaced intervals based on the calculated propensity scores and checks to see if the average propensity scores in the treatment and control groups are comparable. If this assumption is not met, then the algorithm splits the intervals in half and tests again. This process repeats until all the intervals have comparable average propensity scores between the two groups. The program ends by testing for balancing of covariates: the means of each covariate in the treatment and control groups have to be comparable. If the balancing property is satisfied, pscore will notify the user accordingly; otherwise, it will let the user know to try a different set of covariates to match on.
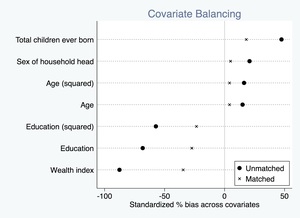
In our case, the treatment variable was receipt of any cash transfer, and we chose to match our sample on age, years of education, wealth index factor score, number of children ever born, and sex of household head. The calculated propensity scores were relatively well-balanced between the two comparison groups as shown in Figure 1, and through matching, we were able to significantly reduce the bias from observable confounding factors (Figure 2).
Using the propensity scores obtained through pscore, we then proceeded to estimate the average treatment effect on the treated (ATT). Because propensity scores are continuous variables, it is highly unlikely that a treated and a control will have the exact same propensity score. As such, we must estimate the ATT using alternative methods that select comparable treated and controls. We chose to use three methods: nearest-neighbor, kernel, and stratification matching. Although each produced slightly different ATT values, their estimates were consistent with one another.
For most of the variables we examined, the KDHS data were usable in their native formats; however, we had to construct a few outcome variables ourselves. We constructed the comprehensive HIV knowledge variable following the definition provided in the official summary report of the 2014 KDHS, which defined this variable as:
“knowing that consistent use of a condom during sexual intercourse and having just one uninfected faithful partner can reduce the chance of getting the AIDS virus, knowing that a healthy-looking person can have the AIDS virus, and rejecting the two most common local misconceptions about transmission or prevention of the AIDS virus” 11
Additionally, we constructed the most recent HIV test 2+ years ago variable by assigning responses of 23 months and lower the value of 0, and responses that were coded as two or more years the value of 1. We constructed the partner 10+ years older variable by subtracting the responding individuals’ ages from their provided ages of their sexual partners and then coding the differences that were less than 10 as 0, and the differences that were 10 or more as 1.
Because most of the outcomes we investigated were binary, we simply coded them as either 0 or 1, with 0 indicating the lack of the particular outcome and 1 indicating the occurrence of the outcome. Therefore, averages of outcomes within the treatment and control groups were the sums of 0s and 1s divided by the respective numbers of observations. Thus, the ATT values we obtained can be interpreted as the differences in the probabilities of observing specific HIV-associated risk behaviors between those who received cash transfers and those who did not.
RESULTS
Survey respondents who did not receive cash transfers were significantly different from those who did. Table 1 displays the complete results of this analysis. Those who did not receive cash transfers were significantly younger (32.44 years vs. 34.36 years, P<0.001), were more likely to be male (31.19% vs. 22.72%, P<0.001), had more years of education (7.9 vs. 4.9, P<0.001), were more likely to be in a household led by a male (68.74% vs. 55.06%, P<0.001), had fewer household members (4.29 vs. 5.47, P<0.001), were more likely to be currently employed (74.43% vs. 60.25%, P<0.001), and were less likely to be illiterate (17.99% vs. 43.56%, P<0.001). Because of all these differences in the treatment and control groups, we proceeded with propensity score matching to generate more comparable groups.
Using propensity score matching, we were able to calculate the average treatment effect on the treated (ATT) for various HIV risk indicators. Table 2 highlights our main results. Those who received cash transfers were less likely to have paid for sex in the past 12 months (ATT: -0.024, P=0.023), more likely to know of a place to get tested (ATT: 0.022, P=0.030), and more likely to have used a condom with their most recent partner (ATT: 0.045, P=0.038). Interestingly, those who received cash transfers were also more likely to have a partner 10 or more years older (ATT: 0.102, P<0.001), less likely to have comprehensive HIV knowledge (ATT: -0.118, P<0.001), and more likely to have a most recent HIV test result that was from two or more years ago (ATT: 0.052, P=0.028).
DISCUSSION AND CONCLUSIONS
The main purpose of this study was to analyze the association of cash transfer programs in Kenya with key HIV risk indicators, including transactional sex, HIV testing, and condom usage. Our results demonstrate that the receipt of unconditional cash transfers is associated with the reduction of some HIV risk indicators, which supports our initial hypothesis. At the same time, though, we observed that the receipt of cash transfers was associated with increases in other risk indicators. These negative results suggest that the marginal increase in household income provided by unconditional cash transfers is not enough to completely counteract the effects of lower levels of health literacy associated with lower levels of wealth. This explanation is reasonable, since many of the various cash transfer programs we examined were not explicitly focused on increasing health literacy or reducing HIV risk. Nevertheless, our positive results are suggestive of the largely protective association between cash transfers and HIV risk.
Although we cannot conclusively establish causation, these results are promising and attest to the far-reaching benefits of cash transfer programs, beyond those conceived at their inception. These results are in line with the findings of other researchers in similar contexts. For example, our finding that cash transfers are associated with a decrease in the probability of engaging in transactional sex is consistent with the results of Cluver et al’s work in South Africa. 12 Kohler and Thornton also support one of our findings: they found a 5.2% increase in the likelihood of condom usage among men who received conditional cash transfers in Malawi 12; quite similar to the 4.5% increase we found in Kenya.
Nonetheless, we should take these findings with caution given the non-random receipt of cash transfers as well as the observational and cross-sectional nature of the data, limiting our ability to estimate causal effects. However, given the nationally representative data and the extensive set of covariates used in our analysis, our study was able to mimic (based on observables) a quasi-experimental design through the use of propensity score matching, and thus make empirical estimations of the relationship between receipt of cash transfers and reduction of certain HIV risk indicators. Further research is needed and should focus on the collection of longitudinal data to allow for more robust causal inference analyses overtime. Additionally, if future iterations of the KDHS include HIV test results along with data on cash transfers, then our work could be extended to measure the association between cash transfers and HIV status. We would initially predict that cash transfer recipients have a lower likelihood of being HIV positive than those who do not receive them.
Acknowledgements: The authors would like to acknowledge the DHS Program for their provision of the data.
Funding: This study was partially supported by the NIH (1R01MH11807501A1); we are grateful to the Population Studies and Training Center (PSTC) at Brown University, which also receives funding from the NIH (P2C HD041020), for general support.
Authorship contributions: KC and OG conceived of and designed the analysis plan. KC carried out the data analysis and wrote the initial draft. SC and OG provided guidance on the analysis and interpretation of results. All authors reviewed and approved the final draft.
Competing interests: The authors completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author), and declare no conflicts of interest.
Correspondence to:
Omar Galárraga, PhD
Department of Health Services, Policy, and Practice
School of Public Health
Brown University
121 South Main Street
Box G-S121-2
Providence
Rhode Island, 02912
USA
[email protected]

.jpg)
.jpg)
