The problem: low and inequitable coverage of those infected with hepatitis C
About 71 million people are affected by chronic hepatitis C (HCV) globally,1 about 80% in LMICs,2 but as of 2016 only 13% of people with chronic HCV had ever received treatment.1 In Morocco it is estimated that more than 350,000 people have chronic HCV infection and there are an estimated 5,000 deaths due to complications of HCV each year.3 The epidemic is no longer growing rapidly, as a result of more effective blood screening and growing harm reduction programs. Nonetheless, there are still an estimated 5,500 new infections each year, and HCV-related morbidity and mortality are expected to increase in the coming years as severe complications of cirrhosis and liver cancer appear among those infected in the past.
At present, only 1,500 patients are being treated for HCV each year with highly effective direct acting antivirals (DAAs) in Morocco. These patients make up only a small fraction of the total infected population and are among the 30% of Moroccans who are covered by mandatory social health insurance (AMO) or have the financial means to purchase DAAs in the private sector. The majority of those infected, who depend on the Ministry of Health for their care, are unable to access these life-saving cures since the Ministry does not yet purchase and supply DAAs to its public healthcare facilities. Previously, some of these patients were treated with pegylated interferon, but most Moroccan doctors and their patients now refuse to use this more toxic and inferior therapy.
Faced with the high burden of HCV, and with the possibility of using inexpensive generic DAAs, the Ministry of Health decided in 2015-16 to develop a national strategic plan (NSP) for hepatitis, assisted by the World Health Organization. This NSP was framed around elimination of HCV by 2030, in line with the global targets adopted by the World Health Assembly in May 2016.4
The NSP spelled out the key interventions and policy actions to achieve HCV elimination. These interventions were modelled on WHO guidance and on intervention packages that have been implemented on a large scale in other middle-income countries, such as Egypt, Mongolia, and Georgia. The interventions included: information campaigns and outreach to improve awareness of the disease; training of health workers in screening, counselling, treatment, and patient monitoring, in order to improve the capacity of the health system to provide HCV services; harm reduction services for people who inject drugs, in order to prevent new infections; vastly expanding testing services with new and less costly rapid tests to find those undiagnosed; strengthening and decentralizing laboratory facilities to support higher loads of viral load and other key tests for streamlining diagnosis and treatment; and procurement and supply of generic versions of direct acting antiviral drugs at low and affordable prices to enable treatment access.5
Rationale for conducting an investment case
In 2017, there was still no clear sign that the Government intended to move ahead to finance and implement the NSP. At this point, the leading HIV/hepatitis NGO in the country, l’Association de lutte contre le SIDA (ACLS), with support from Coalition Plus (C+), a Paris-based global advocacy organization, decided to organize a campaign to accelerate action. As part of this campaign, ALCS and Coalition Plus recognized that while the NSP contained quantitative targets for HCV treatment coverage, there were major gaps in the estimation of costs, financial affordability, expected health impacts, cost-effectiveness, and potential savings to the health sector from implementing the NSP – all important elements in making a persuasive case for financing HCV scale-up for all Moroccans including the poor.
ALCS and Coalition Plus, convinced that such additional epidemiological and economic analysis would be important to inform and strengthen its advocacy, called on our team in early 2017 to work with them to develop a comprehensive “investment case” for HCV treatment. From April to October 2017, our team carried out and presented the results of the investment case analysis to ALCS, Coalition Plus, and the Moroccan Minister of Health. This paper summarizes the methods and results of the investment case, and the policy dialogue that ensued around the trade-offs and benefits of different scale-up scenarios that could guide Morocco towards HCV elimination and a more comprehensive and equitable system for treating HCV. It ends with a discussion of recent events in Morocco and possible future uses for the investment case in strengthening national decision-making on HCV.
The key objective and questions to be answered
As mentioned earlier, the main objective of the analytical modeling exercise was to create an investment case for HCV treatment that could be used to influence program and financing decisions by the Government, with the goal of making treatment more accessible, giving special attention to securing coverage for the 70% of the population not covered by social health insurance (SHI), and thus achieve greater equity in treatment access.
Based on early consultations with ALCS/C+, the Government, and other stakeholders including clinicians, pharmaceutical manufacturers, and insurers, a series of key policy questions emerged, which framed the investment case modeling work. The most fundamental ones included:
-
What would be the likely impact of treatment scale-up on reduced infections, liver disease, and liver-related death for all Moroccans, including the poor?
-
How much would scale-up of HCV treatment cost over the first five years of the NSP and up to 2030?
-
Would the overall price tag of the HCV scale-up be affordable to the Government? What major actions, such as more competitive and lower prices for DAAs or simplification of procedures in the diagnostics cascade, might result in important reductions in cost?
-
Would an HCV treatment program be cost-effective? Would it ultimately save money for the Government as a result of reduced spending to treat liver disease?
METHODS
Developing the investment case approach
The investment case framework has been established as an advocacy and planning policy technique in many disease areas6–9 but is just beginning to be used for hepatitis. The goal of an investment case is to bring together a wide range of analyses on scenario options, disease modeling, costing, cost-effectiveness, budget impact, and financing to create a narrative about how a public health program can be scaled, what will be required to operationalize it, and why it makes sense to prioritize this investment over others. The investment case approach10 sits at the intersection of public health, health economics, and advocacy, in an effort to create a compelling narrative for action backed by rigorous evidence.
There is a small but growing body of country-level investment studies for HCV.11,12 These early examples are beginning to demonstrate the value of this approach for HCV decision-making, but they need to be applied to a wider range of countries while also testing and improving the analytic and modeling tools involved and expanding the effort to collect relevant epidemiological, financial, and economic data. In the case of Morocco, we combined and advanced the use of existing tools and data in order to develop plausible scenarios for decision-making and advocacy (see all appendices in Online Supplementary Document(Online Supplementary Document) for details on our methods, tools, and data for disease modeling, cost estimation, and cost-effectiveness analysis).
Scenario analysis
To best illustrate the range of future policy options, we developed three scenarios in collaboration with stakeholders: 1) the status quo 2) rapid scale-up to elimination in 2030 and 3) gradual scale-up to elimination in 2030.
-
Status quo: This was the counterfactual of no scale-up. It was assumed that the government would maintain current treatment levels at 1,500 patients annually, thus excluding the poor.
-
Rapid scale-up: In accordance with the ambitious commitments from the government, this scenario represented an intensive program scale-up to reach maximum targets in the first five years. These targets mirrored those in the former health minister’s speeches in 2017, and were consistent with the projected coverage in the government’s NSP, which aims for elimination by 2030. This scenario would require increasing the number of patients treated per year by twenty-fold from year one to year five. The peak year of the program would be in 2022. The NSP was in the process of being published when this manuscript was written. The Ministry of Health shared the draft versions of the NSP and authorized its use for this exercise.
-
Gradual scale-up: As an alternative to the Rapid scenario, a second scale-up scenario was modeled. This scenario would still achieve elimination by 2030 but with a slower build up in the early years. Instead of treatment targets peaking in 2022 as the Rapid scenario does, the peak year would not occur until 2029. This scenario would allow additional time to build out a national program and mobilize the needed resources.
Annual treatment targets for each scenario are listed in Appendix S2 in Online Supplementary Document(Online Supplementary Document).
Disease modeling
Disease modeling was conducted in close collaboration with the Center for Disease Analysis (CDA), building on their well-established HCV disease progression Markov model.13,14 CDA has been working with the Ministry of Health since 2016 and its early approximations of treatment targets were used to inform Morocco’s NSP. For this investment case, CDA adapted their model to run the three scenarios described above. Baseline epidemiological parameters were also updated using the most recently available data, including an anti-HCV prevalence of 1.2% and 413,000 anti-HCV positive individuals; a viremic prevalence of 0.9% and 309,000 RNA positive individuals; and 5,600 new infections annually. Disease outcomes of interest included mortality, incidence of new HCV infections, and cases of advanced liver disease, including cirrhosis, decompensated cirrhosis, and hepatocellular carcinoma (HCC). While elimination would be achieved by 2030 in the two scale-up scenarios, disease outcomes was modeled to 2050 to account for delayed onset of HCV disease impacts. Additional information on the CDA model can be found in Appendix S1 in Online Supplementary Document(Online Supplementary Document).
Costing
Financial resource requirements for the scenarios were determined using an activity-based costing approach. Two groups of direct program costs were defined: core program costs and supporting activity costs.
For core program costs, an average cost per person screened, diagnosed, and treated was multiplied by the coverage outputs of the CDA model. The assumed cost per patient screened for anti-HCV was DH50 (US$5) and DH1,300 (US$135) per patient receiving a diagnostic workup (viral load testing, genotyping, and fibrosis staging). Treatment costs of either DH13,500 (US$1,398) or DH27,000 (US$2,795) were applied according to the recommended treatment course for different cirrhosis and genotype profiles. Recommended treatment courses are listed in Appendix S3 of Online Supplementary Document(Online Supplementary Document). All input unit costs were validated by the Morocco Ministry of Health.
For the baseline scenarios, a price of DH13,500 (US$1,398) was assumed for a 12-week course of DAAs, consistent with the price being charged in 2017 by the Moroccan generic manufacturer to social health insurance payers. Since DAA regimens can differ for more advanced patients and different genotypes, an average cost per patient treated was calculated based on epidemiological projections. In testing the impact of “efficiency breakthroughs” (see below) on projected program costs, lower DAA prices of US$500 and US$200 per course were analyzed. We considered these prices in order to understand how sensitive the total program costs were to changes in DAA prices. A price of US$500 per course was selected as an optimistic figure for a country like Morocco with a small market and limited generic competition. The US$200 price per course matched the lowest prices obtained to date in other middle-income countries where there is large demand and significant competition.
Program supporting activity costs (awareness raising, training and human resources, laboratory strengthening, surveillance, information systems, program management) were extracted from Morocco’s NSP, validated, and extrapolated to 2030, based on activity descriptions in the NSP and expert judgment including the views of a local consultant who assisted with the development of the NSP. These activities were assumed to be foundational investments that would not vary with the pace of scale-up. These costs are listed in Appendix S3 of Online Supplementary Document(Online Supplementary Document).
Direct program costs are reported from 2018 to 2030 to represent the financial requirements for the main program scale-up period. Costs are presented in Moroccan Dirham and US dollar. The exchange rate on July 10th, 2017 (9.66 Moroccan dirham to 1 US dollar) was used.
Cost-effectiveness analysis
Incremental cost-effectiveness ratios were calculated for the two scale-up scenarios based on total program costs (all direct program costs) estimated until 2040 and net health benefits (DALYs) to 2050. Any treatment costs beyond 2040 were expected to be negligible since elimination would be achieved by 2030 and the number of remaining infections would decline dramatically. The incremental cost-effectiveness ratios (ICERs) for the scale-up scenarios were compared to relevant benchmarks, including 3 times and 1 times GDP per capita.15 Only a handful of Morocco-specific disease program benchmarks could be found in the literature, limiting our ability to compare the projected HCV program cost-effectiveness to other disease priorities in the country. Costs and benefits were discounted at 3%.16
Return on investment
Return on investment (ROI) of the scale-up scenarios was calculated by comparing the cumulative program costs and benefits of the treatment scale-up scenarios with the status quo. We quantified the benefits of scale-up in two ways: (a) only the assumed savings to the health system from fewer cases of advanced liver disease to treat, and (b) these same savings to the health system plus the monetized value of the DALYs averted. Under both methods, we identified in what year the cumulative costs of the DAA program would become less than the cumulative costs of inaction under the status quo. This point in time was called the “break-even.” Sensitivity analysis on the “break-even” year was conducted for the average annual treatment costs of advanced liver disease.
To calculate savings to the health system, average annual treatment costs for cirrhosis, decompensated cirrhosis, and HCC were multiplied by the modeled number of cases treated per year. Average treatment costs were collected by CDA from a leading Moroccan clinician in 2015 at DH1,700 (US$185) for cirrhosis, DH5,700 (US$618) for decompensated cirrhosis, and DH23,700 (US$2,573) for HCC, in public sector facilities. Private sector costs were reported to be about 1.5-2 times greater. These unit costs for decompensated cirrhosis and HCC were lower than comparable costs from neighboring countries.17–19 To address this uncertainty, we also modeled return on investment for Morocco using more conservative (higher) annual cost estimates of DH24,150 (US$2,500) for caring for decompensated cirrhosis and DH33,810 (US$3,500) for HCC. The CDA model assumes that 80% of all prevalent cases are diagnosed and 100% of those diagnosed are treated. For the monetization of the health benefits, each averted DALY was multiplied by 1 x GDP (US$3,196).
Budget impact/Heath financing analysis
The affordability of the proposed HCV treatment scale-up was examined by comparing the costs estimates for the elimination scenarios with total national health expenditure and Ministry of Health expenditure. Total health expenditure included Morocco’s compulsory social health insurance scheme (AMO), public sector delivery of services, and RAMED, a new insurance scheme to reach the poor.20 Data were based on Morocco’s national health accounts21–25 and the WHO global health expenditure database.26 DAA costs were also analyzed as a percentage of the total medicines budget of the Ministry of Health.27
Efficiency breakthroughs
Hepatitis technologies, prices, and clinical protocols are quickly evolving worldwide. To account for the fact that our baseline assumptions may be conservatively high compared to the expected prices based on future innovations and improvements, we studied four potential factors that could increase HCV treatment efficiency: (a) decrease in price of DAAs from US$1,400 to US$500 or US$200 per cure; (b) elimination of genotyping in the diagnostic workup protocol; (c) reduction in number of viral load tests needed during treatment monitoring from four to two; and (d) the combination of (a), (b), and (c). We studied the effect of these efficiency breakthroughs on total costs, return on investment, and budget impact.
Synthesizing the investment case
The analyses described above were brought together to develop an investment case narrative that captured the extent to which HCV treatment scale-up could reduce disease burden and save lives, while at the same time being an affordable and cost-effective proposition for the Ministry of Health in the short- and long-term. The investment case narrative reflected the analytical rigor of the modeling exercise, but at the same time contained policy takeaways in a distilled form that could be more easily communicated by advocacy partners and interpreted by government decision-makers.
RESULTS
Coverage
The Status Quo scenario assumes that a constant 1,500 individuals would be treated annually. All those treated would be screened opportunistically, with no active screening program initiated. Both the Gradual and Rapid Scale-Up scenarios would involve treating 300,000 individuals from 2018-2030, which would in turn require screening 42 million persons over the same time period. The two scale-up scenarios significantly differ in the timing and intensity of treatment and screening over the thirteen-year period. For the Rapid scenario, 106,810 individuals would be treated and 19.1 million screened in the first five years from 2018-2022. In comparison, under the Gradual scenario, only 19,750 persons would be treated (19% of the Rapid) and 2.7 million screened from 2018-2022 (14% of the Rapid) (Table 1).
Disease impact
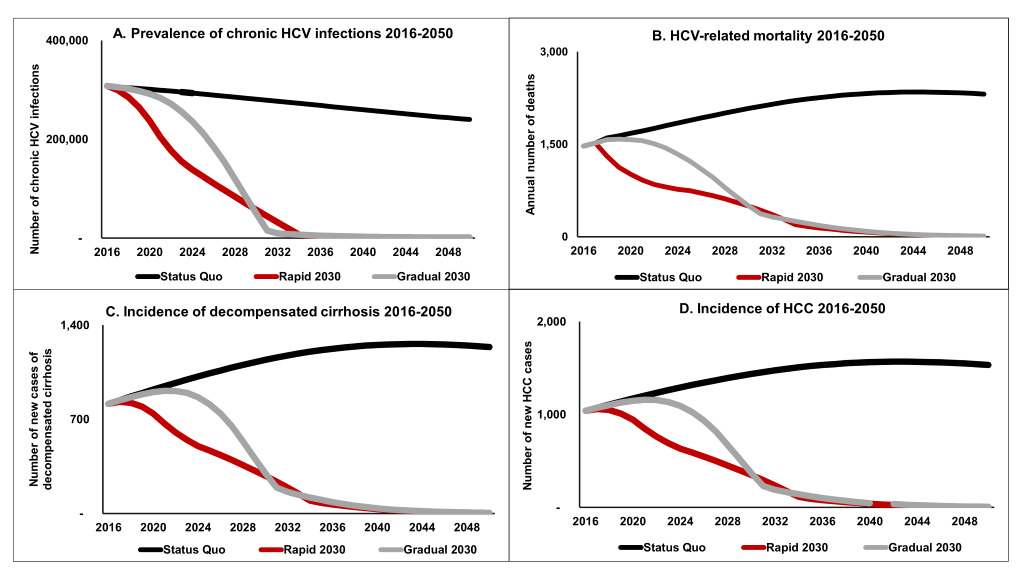
As a result of inaction by the government, the Status Quo scenario would maintain a high level of prevalent HCV virus infections (Figure 1, panel A), with over 240,000 prevalent infections by 2050. The two scale-up scenarios achieve elimination of HCV by 2030 by securing a 65% reduction in HCV-related mortality (from baseline in 2015). From 2018-2050, about 71,000 liver-related deaths would occur under the Status Quo. The Rapid scenario could prevent the majority of these preventable deaths (80%) while the Gradual scenario could avert 72% of them (Figure 1, panel B). Under the Status Quo, 186,000 new infections would occur by 2050. Both scenarios would avert about 140,000 new infections, each preventing about 75% of the infections that would occur in the Status Quo.
Looking at the burden of sequalae due to HCV, the Status Quo would result in 38,705 new cases of decompensated cirrhosis and 48,558 new cases of HCC. Although both scale-up scenarios would significantly reduce this projected burden, extensive treatment in the early years under the Rapid scenario would lead to a greater prevention effort than under the Gradual scenario. Under the Rapid scenario, 37,375 HCC cases (77% of the Status Quo) and 29,814 cases of decompensated cirrhosis (77% of the Status Quo) would be prevented. In comparison, the Gradual scenario would prevent 27,016 HCC cases and 33,849 27,016 decompensated cirrhosis cases (70% of the Status Quo) (Figure 1, panel C and panel D).
Costs
Over 2018-2030, the Rapid scale-up scenario would require DH6.9 billion (US$714 million), compared to DH6.3 billion (US$652 million) for the Gradual scenario. Although these total costs are not significantly different, the implications for the first five years are drastic. Almost half of the total program costs, DH3.2 billion (US$331 million), falls during 2018-2022 under the Rapid scenario, versus only 6% (DH495 million or US$ 51.2 million) of the total program costs for the Gradual scenario during the same five-year period. To implement the Rapid scenario would thus require enormous political commitment from the government. Under the Gradual scenario, the initial demand on the government budget would be significantly lower but would rise over time.
Cost-effectiveness/Return on investment
Compared to the Status Quo, the Gradual scenario was found to be cost-effective at DH13,661 (US$1,414) per DALY averted, about 44% of Morocco’s GDP per capita (US$3,196). The Rapid scale-up would also be cost-effective at DH15,904 (US$1,646) per additional DALY averted compared to the Gradual scenario, still representing about half of Morocco’s GDP per capita. These ICERs suggest that HCV scale-up is highly cost-effective (Table 2).28
Neither scale-up scenario achieves break-even when accounting only for direct program costs of treatment program scale-up and the averted costs of treating advanced liver disease. However, when the DALYs averted under each scenario are monetized, the Rapid scale-up scenario is found to break-even in 2036, while the Gradual scale-up scenario achieves break-even in 2037. When higher costs of treating advanced liver disease were assumed, break-even occurs 1-2 years earlier, in 2034 for the Rapid scenario and 2036 for the Gradual scenario.
Budget impact/Health financing analysis
Overall, the anticipated budget impact of HCV program scale-up appears to be modest, with fiscal space in Morocco seemingly adequate to support the program’s future. Both 2030 elimination scenarios would consume on average 0.9% of projected annual national expenditures for health (with a peak of 2.0% in 2021 for the Rapid scenario and in 2028 for the Gradual scenario) and an of average 3.4% and 3.5% of Ministry of Health spending, respectively. HCV scale-up would however create significant pressure on the Government’s medicines budget. At prices of US$1,400 per cure, DAAs would consume an average of 49% of the projected annual medicines budget for the Rapid scenario and 42% for the Gradual scenario.
Efficiency breakthroughs
Among the factors considered, a reduction in the price of DAAs to US$500 (DH4,830) and US$200 (DH1,932) per course of treatment had the greatest effect in reducing total program costs. For the Rapid scenario, the DAA price reduction to US$500 resulted in a 24% reduction in total costs, while a price of US$200 led to a 43% reduction. Clinical protocol simplifications, including eliminating genotyping and reducing the number of VL tests required, each resulted in 2% reduction in total costs. With a DAA price reduction to US$500 per course, the combined effect of all breakthrough factors occurring simultaneously was a 30% reduction in project program costs from 2018-2030 for the Rapid scenario. With a DAA price of US$200, the combined effect was a reduction of about 50%. For the Gradual scenario, similar effects were observed. These cost savings resulted in break-even being achieved in 2031 for the Rapid scenario and 2034 for the Gradual scenario with the DAA price of US$500, 4-5 years earlier than in the baseline case, and generated break-evens in 2028 and 2032 with the projected DAA price of US$200.
Lower DAA prices would also help alleviate the anticipated impact on the government budget. At US$500 per cure, total program costs would represent less than 0.8% of national health expenditure for both scenarios. The Gradual Scenario would consume 2.4% of the Ministry of Health budget and the Rapid Scenario 2.8%, on average. The Ministry’s medicines budget would also benefit, as lower DAA prices would limit the degree to which DAAs would overwhelm currently projected medicines spending. At US$500 per cure, DAA costs would drop from 50% of the medicines budget to 19% (Gradual) and 26% (Rapid). With an assumed DAA price of US$200, total program costs would absorb 0.5% of national health spending and 1.4-1.7% of the projected health ministry budget, and 5-8% of the medicines budget. This underscores the importance of Morocco using competitive procurement methods in order to obtain lower medicines prices.
DISCUSSION
The overarching purpose behind the development of the Morocco HCV investment case was to use data and modeling to explore the implications of HCV elimination treatment scale-up, including its expected health impact, projected costs, value for money, and financial affordability to the country. Making DAA treatment accessible to the 70% of Moroccans infected with HCV and not covered by social health insurance is justified on ethical grounds. But we sought to further explore the case for investment and appeal to additional motivations of policy makers, by rigorously demonstrating that such a program makes economic sense as well.
Local and international advocates requested the investment case in order to bolster their arguments in favor of a large national effort to eliminate HCV. In the course of undertaking the investment case, government officials also expressed strong interest in our analysis, as they saw it as an important addition to their hepatitis NSP and as a useful input to their decision-making, since the investment case contained estimates of future costs (for budgeting), health benefits, and cost-effectiveness (to persuade the Ministry of Finance to allocate funds for the NSP).
Our overall findings from the investment case are positive and make a strong case for HCV treatment scale-up. Based on our modeling, such scale-up would have major health benefits, averting 51,000-57,000 deaths from HCV by 2050. While the cost of the program is significant, at DH6-7 billion (US$650-715 million) over the next 13 years, the expected cost-effectiveness is good in relation to the standard benchmarks, at less than 0.5 times Morocco’s per capita income. The required investment also appears to be affordable for the public sector, as long as the Ministry of Health prioritizes HCV treatment and scales up in a fiscally sustainable manner. Although the existing medicines budget could be strained, a drop in DAA prices would help to reduce the anticipated pressure on the budget.
The two scale-up scenarios modeled clarify what elimination means in terms of required coverage levels for screening and treatment. Overall, about 300,000 patients will need to be treated by 2030 to achieve elimination, and 42 million people will need to be screened. This will require an ambitious outreach and case-finding effort. Ways to minimize the costs of screening should be a priority, given the large volumes of patients involved.
The Rapid and Gradual scenarios demonstrate that there are multiple paths to achieving the elimination treatment targets, either by embarking on an immediate and steep scale-up or by starting slowly and moving to high levels of coverage as the elimination target date of 2030 approaches. In the Rapid scenario, the weight of the program costs would be incurred right away, peaking in 2022. The Gradual scenario would require less funding in the first five years but would be more demanding on the government budget in the second half of the decade up to 2030.
Although the Gradual scale-up option may have the attraction of giving Morocco more time to build its HCV treatment delivery capacity and mobilize the required financial and human resources, there are important trade-offs in terms of expected health benefits. The Rapid scenario would have the greatest impact in altering the trajectory of the epidemic, averting 80% of the HCV-related deaths as compared to the Status quo. The Gradual scenario would generate significant health benefits, but due to the smaller number of patients treated in the early years, about 25% more preventable liver disease cases and deaths would occur in the long run than under the Rapid scenario.
The Gradual and Rapid scale-up scenarios appear to be justified on grounds of cost-effectiveness, with ICERs of US$1,414 and USA$1,646 per DALY averted, respectively. When the savings from averting advanced liver disease and the value of the years of life gained are combined, both scale-up scenarios achieve break-even about 15 years from the time of the program initiation. This analysis suggests that the economic costs of scale-up are less than that of inaction (status quo). This is impressive, given that the onset of most sequalae due to HCV occurs 20 years or more from the time of infection. Furthermore, given the uncertainties surrounding the costs of treating decompensated cirrhosis and liver cancer in Morocco, and the potential for cost-saving breakthroughs such as lower DAA prices, economic break-even could occur 5-8 years earlier than shown in the baseline scenarios.
These economic arguments bolster the case for Morocco to explore ways to create the needed fiscal space for HCV scale-up to reach all of its citizens, including the 35% of the population eligible for RAMED but not yet accessing DAA treatment, plus the remaining 35% with no coverage. Projected costs for HCV would represent about 3.5% of estimated future Ministry of Health funding, a significant share when there are many competing priorities, but also potentially a level of investment that the country can successfully and sustainably absorb.
This study incorporated the best available data and state of the art tools for HCV modeling and analysis, and used multiple scenarios and sensitivity tests to address areas of uncertainty. The findings presented here thus provide a solid basis for policy and investment decisions. Nevertheless, there are important limitations to our work. As is the case in many countries, the data on HCV disease burden in Morocco, which can be gleaned from surveillance and reporting systems, are still poor. The data on HCV epidemiology collected by CDA and used in our modeling represents the best data currently available, but more needs to be done to improve the quality of such data. The CDA model itself also has some limitations, including the fact that it models incidence reductions as a result of reductions in the number of untreated chronic infections, rather than being fully dynamic. At the time of this analysis, HCV modeling was still in its infancy, and the next generation of tools will likely include dynamic transmission models. However, given the low rates of new infection in Morocco, the lack of a dynamic model does not substantially affect our findings.
Our assumptions on unit costs also contain limitations. The costs of treating advanced liver disease (cirrhosis and liver cancer) in Morocco came from a single source interviewed by CDA. A broader survey of health facilities, public and private, would be useful, as was done in other countries such as The Gambia.29 ALCS is currently working on this. While unit costs for key inputs such as HCV test kits and laboratory analyses were drawn from official price lists of the Institut Pasteur and the National Hygiene Institute and are therefore reliable, unit costs for supporting activities such as awareness raising, training, and laboratory strengthening were either taken from the NSP or were based on the knowledge and experience of local experts. Further validation of these unit costs would be desirable in the future.
The financial and economic analyses conducted for the study are from a public-sector perspective, focusing on Ministry of Health plans and activities. It was assumed that those covered by social health insurance (AMO) will continue to be eligible for DAA treatment and that the main gap to be filled is by the Ministry of Health on behalf of the relatively less well-off households. If the AMO system expands to cover a larger fraction of the Moroccan population as part of the Government’s commitment to achieving universal health coverage, the total cost of the HCV elimination program will not change but the proportions covered by AMO and the Ministry of Health will need to be modified. Policy coherence between AMO and Ministry of Health on HCV will be essential for the success of a national HCV elimination effort.
From a process perspective, one of the most important benefits of developing the investment case narrative was to create a shared narrative between government policy makers and advocates on the value of HCV elimination, and to promote a common evidence-based platform for dialogue and action. The investment case was presented to the Moroccan Health Minister and his senior leadership in September 2017. The Ministry has committed to putting more than 6,500 public sector (non-AMO) patients on treatment in 2018-19, over four times the current number treated annually. The 2017 tender was cancelled but the new one – which should be launched by end of 2018 – will aim to increase competition and obtain lower prices. The Ministry has also agreed to pursue a reduction in the number of viral load tests required in treatment protocols, one of the efficiency breakthrough factors modeled for the investment case. The leading national advocacy organization, ALCS, featured the HCV investment case at its annual conference in January 2018 and has reiterated its commitment to strong advocacy and continued dialogue with the government to stimulate the launch of the national HCV treatment program.
CONCLUSION
The Morocco HCV investment case exercise described here demonstrates how the use of evidence and analysis to model HCV treatment scale-up can help support advocacy and guide investment decisions. Advocates can use this information to highlight the costs of inaction and emphasize the mortality and morbidity consequences for those who continue to be denied treatment access. The government can simultaneously draw upon this information to estimate the required financial resources and feasibility of implementing an HCV elimination program, and to assess the anticipated health benefits relative to the investment involved.
The investment case can thus be a powerful tool in specifically advocating for the well-being of the most vulnerable. Access to HCV treatment is a fundamental equity issue in Morocco, as well as a public health challenge. Although the better-off already have access to DAA treatment via the AMO system and by paying out of pocket in the private sector, DAA treatment for the uninsured and most vulnerable remains in the balance. These vulnerable groups often do not have a voice in policy making, but ALCS and other advocates are using the investment case to ensure that the interests of the poor are strongly represented. The investment case provides the evidence needed to support a broader expansion of HCV treatment that can benefit all Moroccans and universalization of access to DAAs on the road to country-wide elimination.
Acknowledgements
The authors are grateful to the Moroccan Ministry of Health for their help in data collection and validation and for reviewing and giving feedback on our modeling results. They wish to acknowledge Jonathan Schmelzer and Homie Razavi at the Center for Disease Analysis for collaborating on the disease modeling and costing and Dr. Benmamoun Abderrahmane for his assistance in data collection. The authors also wish to recognize the World Health Organization for its assistance and advice at various stages of the project.
Funding
The project was funded by Coalition Plus using a grant from Unitaid.
Authorship contributions
RH directed the project and drafted and edited the manuscript; MK synthesized main conclusions, conducted financing analyses, and reviewed the final paper; SR conducted the cost-effectiveness and return on investment analyses and advised on scenario design and disease modeling; JLK assisted in data collaboration and scenario design and reviewed intermediate products and the final paper; CP assisted in developing efficiency breakthrough scenarios and reviewed intermediate products; MS collected data and assisted with scenario design; HH advised all phases of the project, liaised with the Ministry of Health, and reviewed intermediate products and the final paper; LH supported the technical analyses, synthesized findings, and drafted and revised the paper.
Competing interests
The authors completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author), and declare no conflicts of interest.
Correspondence to:
Lindsey Hiebert, MPH
Pharos Global Health
780 Boylston St Ste 16J
Boston, Massachusetts
USA 02120
[email protected]