Globally, cervical cancer is the third most common cancer in women and the second most frequent cause of cancer death with the highest burden found in developing areas of the world.1 Human papillomavirus (HPV) infection, primarily oncogenic types HPV 16 and 18, is the principal cause of nearly all cervical cancers.2 Even with the availability of highly efficacious prophylactic HPV vaccines, screening remains an important component of cervical cancer prevention. In many developing countries, however, screening is underutilized and cervical cancer remains a major public health challenge.3,4 The Federated States of Micronesia (FSM) is comprised of 607 volcanic islands and atolls scattered over 1 million square miles of the Northwestern Pacific Ocean (WHO, 2011). FSM is one of the most resource-limited US Affiliated Pacific Island (USAPI) jurisdictions. Yap State, FSM has a population of approximately 12,000 people living on 22 inhabited small islands and atolls spread across 500 square miles of Western Pacific ocean (Figure 1).
Micronesian women throughout the Pacific have among the highest rates of cervical cancer in the world and often present with late stage disease. The incidence of cervical cancer in Yap is over twice that of the U.S. and most cases are diagnosed at advanced stages.4 The high burden of cervical cancer in Yap is consistent with low levels of screening, which remain at less than 40% throughout the FSM.5 Major barriers to cervical cancer screening in Yap include geography, lack of trained personnel, limited clinical resources, as well as issues of cultural and personal acceptability.5 Primary health care is provided through a hospital and public health clinics on the main Yap island and, for the outer islands, through small health dispensaries run by health assistants and equipped with variable electricity and limited supplies and medication. Cervical cancer screening, largely comprised of cytology (Pap testing) and visual inspection with acetic acid (VIA), is available on the main island and, sporadically, on the outer islands by traveling public health teams.5 Follow-up colposcopy and biopsy as well as treatment for precancerous and early stage cervical cancer are also available on the main island with more advanced stage cancers referred to medical facilities off-island.5 For low-resource communities like Yap, the need for more culturally-, resource-, and health workforce-appropriate methods of cervical cancer screening has been recognized.6 The need for alternatives screening approaches has also been recognized in high resource settings such as the U.S. where over half of cervical cancers are diagnosed in women who are unscreened despite having access to health care.7
HPV DNA testing has been shown to be effective for cervical cancer screening when used as an adjunct to cytology or as a primary test with similar or better sensitivity for the detection of precancerous lesions compared to cytology alone.8–10 Nonetheless, the improved sensitivity offered by HPV DNA testing to supplement or to replace cervical cytology does not address current barriers to cervical cancer screening. Similar to Pap smear collection, current methods for the collection of samples for HPV DNA testing require a trained clinician to directly sample the cervix.
Non-invasive, self-collection sampling strategies which are reliable, efficient, and acceptable have the potential to address current barriers to cervical cancer screening in underserved communities. Self-sampling methods for HPV DNA testing are generally more acceptable and preferable to women compared to collection methods performed by a clinician.11 Evaluation of HPV self-sampling methods has largely focused on the collection of cervical/vaginal or vaginal samples using swabs, brushes, tampons, or lavage.11 In a study of HPV transmission between male-female partners, we found urine to be a good proxy for cervical HPV infection.12 In a meta-analysis, urine was found to be generally accurate for the detection of cervical HPV DNA.13 However, few studies have also compared urine and cervical samples for the prediction of cervical cytologic outcomes.14
METHODS
Objectives
A randomized controlled trial was conducted in the state of Yap in the FSM. The purpose of the project was to evaluate the detection of HPV DNA in self-collected urine compared to clinician-collected cervical cell samples.
Study settings and study subjects
The study was approved by the Western Institutional Review Board. Study participants were enrolled through six Wa’ab community clinics located throughout the region. Written informed consent was obtained from all participants who were enrolled between March-May 2016. Eligible subjects included women aged 21-65 who had not had a hysterectomy, were not currently pregnant, and either who had not been screened within the past 3 years or had abnormal screening results within the past 3 years. The latter criteria based on screening history was intended to target high-risk populations with a high prevalence of HPV. Following completion of the study visit, participants were each provided with a tote bag as a token of appreciation.
Trial design
Study subjects were randomized into one of two groups to account for the potential influence of the order of sampling procedures on HPV DNA detection: 1) Cervical sampling by a trained clinician followed by self-collection of urine; 2) Self-collection of urine followed by clinician-collected cervical sampling. Randomization was based on sequential enrollment into the study at each of the six clinics.
Specimen collection
At each study site, cervical cell specimens were collected by trained clinicians in private examination rooms. A sterile cytobrush was used to sample the endocervical canal and transformation zone then placed into liquid cytology collection media (ThinPrep, Hologic, Inc, Marlborough, Massachusetts, USA). Urine specimens were collected by the participant in a private restroom using a labeled sterile collection cup. Individuals were instructed to collect up to 30 mL of first-void urine. Cervical cell and urine samples were stored at 4 degrees Celsius until they were transported to a central facility on the main Yap island where they were packed and shipped on ice to Honolulu, Hawaii, U.S.A.
Interviewer-administered survey
Interviews were conducted and medical records reviewed to collect demographic characteristics, cervical screening and HPV vaccine history, sexual history, and medical conditions and heath behaviors. The survey also addressed the acceptability of the cervical sampling and urine collection procedures; these results were recently reported.15
Cervical cytologic evaluation
Cervical specimens in the liquid cytology media were processed for cytologic evaluation at a College of American Pathologists (CAP)-certified pathology laboratory in Honolulu, Hawaii. Papanicolaou (Pap) smear stained slides were read by board-certified cytotechnologists using standard cervical cytology criteria based on the Bethesda system.16 Abnormal results were confirmed by a board-certified pathologist. Diagnostic follow-up for abnormal cytology results, including follow-up colposcopy and biopsy, were in accordance with the recommendations of the American Society for Colposcopy and Cervical Pathology.17
For a subset of women with abnormal cytology, liquid cytology specimens underwent reflex HPV testing through the Honolulu pathology laboratory. The Roche Cobas 4800 system (Roche Molecular Systems, Inc.) was used for real-time PCR using group probes for high-risk HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 and individual probes for HPV 16 and HPV 18 (for specimens positive by group probe).
HPV DNA testing
HPV DNA testing of cervical and urine specimens was conducted the University of Hawaii Cancer Center in Honolulu, Hawaii where liquid cytology specimens were sent following cytologic evaluation and reflex testing. Following DNA isolation, specimens were evaluated for HPV DNA using a PCR-based assay to target a consensus region of the HPV L1 gene. Amplicons were genotyped with the Linear Array HPV Genotyping Test (LA, Roche Diagnostics, Indianapolis, IN) which distinguishes 37 HPV genotypes (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52 (XR), 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, IS39). Human beta-globin PCR was included as a measure of sample sufficiency. Samples negative for beta-globin were considered inadequate and were excluded from the statistical analyses.
p16 in urine cytology
Aliquots of urine specimens were concentrated on glass slides using a cytospin. Slides were stained with a p16 mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (dilution 1:400) according to the manufacturer’s specifications. Slides were read by a study pathologist who was blinded to the HPV status of cases. p16 was classified as positive or negative based on any nuclear and/or cytoplasmic staining.
Statistical analysis
SAS software version 9.4 (SAS Institute, Inc., Cary, North Carolina, USA) was used for analyses of data. HPV genotypes were grouped as any HPV and high-risk HPV. HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 were classified as high-risk (oncogenic).18 (Non-oncogenic types and HPV types of undetermined risk status included HPV 6, 11, 26, 34, 40, 42, 44, 53, 54, 55, 61, 62, 64, 66, 67, 70, 71, 72, 73, 81, 82, 83, 84, and 89). Samples positive for 1 or more high-risk genotypes with or without other genotypes were classified as oncogenic, or high-risk.
Agreement of HPV status between self-collected urine and clinician-collected cervical samples was measured by percent agreement and Cohen’s Kappa and McNemar statistics.19 Kappa values were defined as ≤0 (no agreement); 0.01-0.20 (slight agreement); 0.21-0.40 (fair agreement); 0.41–0.60 (moderate agreement); 0.61-0.80 (substantial agreement); (0.81-1.00) excellent agreement.19 The sensitivity and specificity of urine for the prediction of cervical high-risk HPV were evaluated. The sensitivities and specificities of high-risk HPV in both urine and cervical samples for the prediction of cervical cytology were also evaluated. Comparisons between categorical variables utilized the χ2 statistic. All tests were two-sided, and P<0.05 was considered statistically significant.
RESULTS
Study population
A total of 217 women aged 21-65 years were enrolled (Table 1). Five percent of women had prior HPV vaccination (at least one dose) and 59.5% had prior cervical cancer screening via Pap smear and/or visual inspection with acetic acid (VIA). Among those previously screened, 17.1% had abnormal cytology; 3 women had a history of biopsy-confirmed CIN. Sexually transmitted disease history was positive for 17.5% of participants. Family history of cancer was reported by 45.2% of subjects; cervical cancer was the 4th most frequent (data not shown). Two-thirds of women reported 2-9 male sexual partners in their lifetime and over two-thirds reported never using a condom during vaginal intercourse.
Cervical cytology and histopathology
Cervical cytology was normal for 83% of women; abnormal cytology included atypical cells of unknown significance (ASCUS) (14%), low-grade squamous intraepithelial lesions (LSIL) (1.4%) and high-grade squamous intraepithelial lesions (HSIL) (1.8%). Biopsy for follow-up of abnormal cytology was completed for 13 women. Histologically-confirmed invasive cervical cancer was diagnosed in two women, carcinoma in situ in two, CIN II/III in 2, and CIN III in five; two had normal (negative) biopsies.
Sample sufficiency
Overall, 97.7% of self-collected urine specimens were sufficient compared to 98.6% of cervical specimens (P=0.0003) (Table 2). The sufficiency of urine and cervical samples did not significantly vary (P≥0.05) by the order of sample collection, age, clinic site, urine pH, or time since last urination.
HPV DNA detection
HPV DNA (any genotype) was detected in 27.8% of urine samples and 38.3% of cervical samples (P<0.0001) (Table 2). High-risk HPV was detected in 15.1% of urine and 23.8% of cervical samples (P<0.0001). A total of 24 distinct HPV genotypes were detected in urine and 29 genotypes in cervical samples (Figure 2). The most frequently detected types in urine were oncogenic HPV 51, 58, and 68 and other HPV 54, 62, and 72. For cervical samples, the most frequently observed types were oncogenic HPV 16, 31, 51, 52, 58 and other HPV 62. Multiple genotypes were detected in 36% and 31% of HPV positive urine and cervical samples, respectively.
HPV DNA detection was compared by study and participant variables (data not shown). Detection of HPV DNA (any genotype) in both urine and cervical samples did not vary by the order of collection, clinic site, urine pH, time since last urination, number of sexual partners, or condom use (P≥0.05 for all). Urine HPV DNA detection by age group did not vary by age: 35.1% (20-29 yrs.), 30.8% (30-39), 21.7% (40-49), and 26% (50 and over) (P=0.48). In contrast, cervical HPV DNA detection decreased with age: 58.3% (20-29), 40% (30-39), 32.3% (40-49), and 29.4% (50 and over) (P=0.03). Cervical HPV DNA detection also varied by alcohol and betel nut use. HPV DNA was detected in 50.5% of current alcohol drinkers compared to 28.6% of non-drinkers (P=0.001). HPV DNA was detected in cervical samples of 40.4% of betel nut chewers compared to 15.8% of non-chewers (P=0.04). Urine HPV detection did not vary by alcohol or betel nut use. Although there was no variation of HPV DNA by hypertension status, there was some variation of HPV DNA detection by use of hypertension medication. Urine HPV DNA was not detected among any women who used hypertension medication compared to 29% of those non-users although the difference was not statistically significant (P=0.06). Of the 11 women who had a history of HPV vaccination, 5 were HPV positive in urine and cervical samples for genotypes other than quadrivalent vaccine-covered types (HPV 6, 11, 16, and 18). (Normal cervical cytology was observed in 10 of 11 HPV vaccinated women; 1 was ASCUS).
Agreement of HPV DNA in paired urine and cervical samples
Overall, HPV DNA detection in paired urine and cervical samples showed moderate agreement (Kappa=0.55, 95% CI=0.43-0.66) (Table 3). Genotype concordance (partial or complete) was 81.6% for HPV-positive urine-cervical pairs. Agreement was similar for samples positive for high-risk genotypes (with or without concurrent presence of other types) (Kappa=0.54, 95% CI=0.40-0.68). High-risk HPV agreement was moderate when urine samples were collected first (Kappa=0.57, 95% CI=0.39-0.76) as well as when cervical samples were collected first (Kappa=0.51, 95% CI=0.31 - 0.71). Agreement between paired urine and cervical samples substantially varied by age. For high-risk HPV, agreement was moderate for women age 20-39 (Kappa=0.45 95% CI=0.25-0.64). Among women ages 40 and older, high-risk HPV agreement was substantial (Kappa=0.65, 95% CI=0.46-0.85).
Agreement varied across the six Yap study sites ranging from fair levels of agreement (Kappa=0.29, 95% CI=-0.11-0.70) to substantial levels of agreement (Kappa=0.78, 95% CI=0.50-1.00). Age is unlikely to have influenced HPV agreement by study site as the age distribution of study subjects across study sites did not significantly vary (P=0.11).
Given the variation in HPV DNA detection in cervical samples by alcohol and betel nut use, agreement between paired urine and cervical samples was compared by use of these substances. Agreement did not vary by betel nut use but significantly varied by alcohol use. Among current drinkers, agreement between paired urine and cervical samples was moderate (Kappa=0.43 95% CI=0.26-0.60) while among non-drinkers, agreement was substantial (Kappa=0.65, 95% CI=0.49-0.81). Agreement could not be compared by hypertension medication use as urine HPV DNA was not detected among any users.
Reflex HPV testing
Reflex HPV testing of liquid cytology cervical specimens was conducted for 29 individuals with abnormal cytology. Reflex testing yielded 14 of 29 positive for HPV 16/18/31/33/35/39/ 45/51/52/56/58/59/68; 1 result was indeterminate. One of the 14 cases were positive for HPV 18 upon additional testing for HPV 16 and HPV 18. For the 29 cases undergoing reflex HPV testing, compared to with samples tested by the Roche linear array, agreement in high-risk HPV DNA detection was excellent for cervical samples (Kappa=0.85, 95% CI=0.65-1.00) and substantial for urine (Kappa=0.64, 95% CI=0.37-0.92).
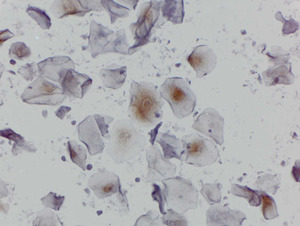
p16 in urine
p16 was expressed in 45% of urine cytology samples including strong expression in koilocytes of a sub-set of cases (Figure 3). However, p16 was poorly correlated with urine HPV positivity (percent agreement 57.2% any HPV; 55.3% high-risk HPV). p16 expression also did not correlate with cervical cytologic status (data not shown).
Urine sensitivity and specificity for cervical HPV DNA and abnormal cervical cytology
The sensitivity and specificity of urine high-risk HPV for prediction of cervical high-risk HPV and abnormal cytology were evaluated (Table 4). (Abnormal cytology including ASCUS, LSIL, and HSIL were combined as the numbers were too few for separate evaluation.) For the prediction of cervical high-risk HPV DNA, the sensitivity of urine was 51.0% (95% CI=37%-65%) and specificity was 96.2% (95% CI=92.0%-99.0%). For ASCUS/LSIL/HSIL, the sensitivity of high-risk HPV in urine (47.4%, 31.0%-64.2%) was less than that of cervical HPV DNA (57.9%, 95% CI=40.8%-73.7%). In contrast, the specificity of high-risk HPV in urine (92.0%, 95% CI=86.9%-95.5%) was greater than that of cervical HPV (83.5, 95% CI=77.2%-88.7%).
DISCUSSION
In this randomized controlled trial, self-collected urine was generally inferior to clinician-collected cervical samples for the detection of cervical HPV DNA. HPV DNA detection was lower in urine compared to cervical samples and agreement was moderate between paired samples. The observed sensitivity of urine for the detection of cervical HPV (59.8%) in this study was on the lower end of sensitivity demonstrated in other studies evaluating urine which ranged from 53% to 99%.13,20 In contrast, the specificity (92.2%) was on the higher end of the range of specificities (38% to 99%). Nonetheless, comparisons across studies are limited by the variation in study populations, age distribution, collection methods, and laboratory assays.13
For the prediction of abnormal cytology, HPV measured in urine was less sensitive but more specific than clinician-collected cervical samples.
Our findings support that urine HPV detection may be most clinically useful in older women. Agreement in HPV DNA detection between paired urine and cervical samples was substantial among older women. This is consistent with evidence that HPV DNA testing as primary screening tool or as co-test with cytology is most suitable for women aged 30 years and older.8–10 Sample size limitations did not permit separate age group comparisons of urine and cervical HPV DNA for the prediction of cervical lesions.
Agreement between paired urine and cervical samples and adequacy of urine specimens did not vary by the order of collection indicating that sufficient cervical cells remained to be shed into urine following directed sampling. Moreover, urine HPV was not influenced by the time since last urination suggesting the continuous shedding of HPV-infected cervical cells.
The wide variation across clinical sites underscores the potential influence of logistical factors that may have influenced the integrity of samples. Clinical sites across Yap included more resourced facilities on the main island as well as the less resourced, smaller dispensaries. Consequently, there may have been variation in the quality of clinician- and self-collected samples. Although only a fraction of all samples were insufficient, they included significantly more urine than cervical specimens. There was no way to verify that the participants fully complied with the instructions for self-collection including the collection of first-void rather than mid-stream urine, the former of which is superior for the detection of HPV.13,20
Sample integrity may also have been influenced by other factors related to specimen collection, storage, and processing. In contrast to cervical samples collected into liquid cytology media, urine samples were collected without the use of a DNA-preserving media due to concerns of the spillage of media during the self-collection process. Degradation of viral DNA in urine samples in the absence of a stabilizing media has been reported.21 Urine and cytology specimens collected at the six sites were transported to a central facility on the main Yap island prior to shipment to the testing laboratory in Honolulu. It is possible that variable specimen handing practices across clinical sites and transport delays may also have affected sample integrity and subsequent measures of specimen sufficiency and HPV DNA.
Concordance of genotypes was observed among the majority of samples positive in both urine and cervical paired samples. This underscores that urine and cervical samples were largely measuring the same cervical infection. Conversely, our findings provide some evidence that HPV detected in urine and cervical samples to some extent represented different anatomical sources. Collectively, the most frequently detected genotypes were somewhat different in urine and cervical samples. HPV in urine may represent viral infection from cells shed by the vagina and vulva in addition to the cervix. There is some evidence that genotypes trophic to the lower genital tract are not entirely consistent with cervical HPV genotypes.22
That urine HPV is not specific to the cervical infection is also supported by observed differences in age distributions. Cervical HPV DNA significantly varied with age with the highest prevalence in young women while urine HPV prevalence did not vary with age. As we and others have observed in U.S. and other populations, cervical HPV prevalence is strongly correlated with age with the highest prevalence in young women and declining with increasing age.23,24 Cervical HPV has also been shown to be correlated with number of sexual partners across population.25 Interestingly, we observed that HPV detection in both urine and cervical samples did not vary by number of sexual partners. This might reflect a limited exposure range in this generally high-risk study population.
In the cervix, elevated expression of p16(INK4A), or p16, a cyclin-dependent kinase-4 inhibitor, is strongly correlated with HPV positivity and the presence of high-grade lesions.26 p16 expression in urine did not correlate with urine HPV or cervical cytology. Nonetheless, p16 was strongly expressed in koilocytes, which were found in a limited number of urine specimens. The detection of koilocytes in urine does lend support to the notion that HPV-infected cells of gynecologic origin are shed into the urine. The presence of urinary koilocytes has been reported in an immunosuppressed patient with cervical dysplasia and condylomatous lesions in the vulva, vagina, and cervix.27
Our results provide some evidence that the detection of HPV in urine and cervical samples and agreement between the two media is influenced by substance use. Agreement of HPV DNA detection in urine and cervical samples was substantial among non-drinkers but moderate among drinkers. HPV prevalence in cervical samples was significantly higher in drinkers compared to non-drinkers suggesting that this variation accounted for the better agreement of the two media among non-drinkers. Interestingly, HPV DNA detection in cervical samples also significantly varied by betel nut use with higher prevalence among daily users than non-users. Urine HPV did not vary by alcohol or betel nut use. Interestingly, none of the women taking medication for hypertension were positive for urine HPV. It is possible albeit speculative that the detection of urine HPV is impeded by the diuretic effects of such medications which result in increased urine volume.
It should be noted that the Roche Linear Array assay utilized for the present study is not among the U.S. FDA-approved HPV assays that have been validated as a primary screening tool. Unlike other assays which utilize group probes, the Linear Array allows for discrimination of 37 individual HPV genotypes and has been extensively used for research purposes in the U.S. and worldwide. The Linear Array has been shown to be comparable to other HPV assays including the FDA-approved Cobas 4800 test. We observed excellent agreement between cervical samples tested in the linear array and the subset which underwent reflex testing with the Cobas 4800 test. In fact, high-risk HPV agreement was substantial between reflex tested cervical samples and urine samples although this was based on a very small sample. Other evaluations have also shown the linear array assay to correlate well with the Cobas 4800 test28 as well as with the FDA-approved Hybrid Capture 2 assay.29
In a number of developing countries where screening is available, screening rates remain low and a high burden cervical cancer persists.3 For low-resource settings such as Yap, there is a need for alternative screening strategies. The development of urine-based or other self-collection strategies for cervical cancer screening has the potential to transform prevention worldwide including in low-resource populations as well as underserved communities within developed areas of the world. Such strategies may include primary screening in accordance with current age-based clinical guidelines and would be particularly useful in populations where clinically-collected cervical samples cannot be obtained. Urine or other self-collected sampling could also be the basis of novel strategies such as the identification of high-risk women through mass HPV testing of self-collected samples followed by targeted cytologic and HPV screening of high-risk HPV-positive older females. Self-collected samples may also be useful for follow-up of patients with abnormal cytology incorporating periodic HPV testing in order to identify those with persistent high-risk infection as these individuals bear the greatest risk for neoplastic progression.18 Such non-invasive follow up could reduce unnecessary colposcopy and biopsy procedures along with their associated medical and psychosocial sequelae, costs, and resources. Urine-based or other self-collected HPV testing may also be useful for monitoring the uptake and effectiveness of prophylactic HPV vaccination across populations.30 This is particularly relevant in adolescent female populations for which invasive cervical sampling is not appropriate.
CONCLUSIONS
Urine is less sensitive but more specific than directed cervical sampling for detection of cytologic abnormalities. Although the limited study population restricts our findings, our study provides evidence that urine may have utility for cervical screening, particularly in older populations of women when clinician-collected samples cannot be obtained. Confirmation of our study results in larger studies of low- and high-risk populations is needed.
Acknowledgements
We would like to acknowledge the project steering committee in Yap, comprised of public health and medical leaders, including key staff from the Yap Hospital Lab, MCH and Family Planning programs, Wa`ab Community Health Centers, Yap Cancer Program, Fais Outer Island Dispensary staff and local health board. Thank you to the women of Yap who participated in this first randomized controlled trial in the FSM.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Centers for Disease Control and Prevention.
Funding
National Cancer Institute of the National Institutes of Health under award number NCI=3P30CA071789-16S3 (BYH, ACT, MR AG, XZ, AS, AY, LEBL), as well as the Centers for Disease Control and Prevention awards: CDC U58 DP000976 and U58 DP003906 Pacific Regional Central Cancer Registry (LEBL) and CDC U58 DP000779 FSM National Comprehensive Cancer Control Program (ACT, MR, AA).
Competing interests
The authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no conflict of interest.
Correspondence to:
Brenda Y. Hernandez
University of Hawaii Cancer Center
701 Ilalo Street
Honolulu, Hawaii, United States of America
[email protected]

_is_comprised_of_607_islands_and_atolls_scattered_.png)

_is_comprised_of_607_islands_and_atolls_scattered_.png)

