Vaginal rings that can deliver an antiretroviral (ARV) drug for preventing HIV infection, separately or in combination with agents for preventing pregnancy or sexually transmitted infections (STIs), are under development.1–3 HIV prevention researchers anticipate that vaginal delivery of ARV drugs by long-acting (i.e., >28-day) methods will help avoid various adherence issues observed for some women with oral daily4 or peri-coitally dosed pre-exposure prophylaxis (PrEP).5 Contraceptive vaginal rings (CVRs) may offer valuable insights on users’ experiences, including potential adherence challenges associated with use of this technology, in particular among first-time users.
Worldwide, an overall low estimated prevalence (0.1%) of vaginal contraceptive methods, including CVRs, among women 15 to 49 years of age6 raises concerns about potential uptake (i.e., action taken to initiate use) and use of a multipurpose ring. Explanations for low uptake of CVRs include challenges with vaginal insertion, foreign-body sensation,7 sexual interference,8 and concerns about the ring getting lost inside the body.9 Poor CVR promotion, cost, and short duration of use (e.g., 21-day use cycle) further account for CVR uptake obstacles.10 While high satisfaction, tolerability, favorable bleeding control, and adherence have been reported by women initiating CVR use,3 a continuation rate of 26% after 6 months by new CVR users has been observed, which is even lower than the 29% reported for combined oral contraceptives.11
Adherence in clinical trials is subject to overestimation given challenges with measuring product use, both behaviorally and biologically.12 Nonetheless, adherence in a trial is expected to be as good as or greater than “real world” use, where social, structural, and behavioral interventions may need to be coupled with biomedical ones and directed at providers and target populations alike.13 Women’s empowerment, defined as the “ability to make effective choices and to transform these choices into desired outcomes” as opposed to merely following prescriptive conventions,14 is a critical component in acceptability, satisfaction, and adherence of female-focused health interventions, including contraception. Women’s empowerment, which is influenced by individual, relational, and social determinants of health (e.g., socioeconomic status, education, gender dynamics, healthcare access, availability of methods, etc.), intersects with perceptions related to product properties, including the safety profile.
Given the seemingly apparent reasons that women would be interested in CVR use, it stands to reason that to optimize adherence, we need a better understanding of women’s experiences with this technology, in particular barriers to use. To address this issue, we examined the association of CVR non-adherence with user dissatisfaction, tolerability, demographic, and behavior variables among first-time users of this technology in Kisumu, Kenya. Of note, no CVR is licensed currently for use in Kenya, and the target population in this setting was predominantly naïve to CVRs. Our analysis sample included women who had at least one follow-up monthly visit post-CVR insertion. Two additional sub-analyses were performed for participants who provided follow-up behavioral data or were selected purposively for assessing residual progestin and estrogen levels in returned CVRs.
MATERIALS AND METHODS
Between April 2014 and August 2015, an open-label single-group study of NuvaRing® (Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc, Kenilworth, NJ, USA) use was conducted in Kisumu, Kenya. In brief, a convenience sample of women was recruited from family planning and reproductive health clinics within Kisumu County via help of 10 community health volunteers and participant word-of-mouth referrals without incentives. We enrolled women who were 18-34 years of age, resided within a 150-kilometer radius of Kisumu City with no plans for relocation in the next 12 months, were fluent in English, Swahili, or Dholuo, provided documentation of depot medroxyprogesterone acetate (DMPA) or oral contraceptive pills (OCPs) use in the past three months, self-reported ≥2 episodes of vaginal intercourse on different days in the past 30 days at screening, tested negative for pregnancy and HIV, had no current or history of known medical contraindications for CVR use, were not breastfeeding or within three months of parturition at screening, and were prepared to use the CVR for six months in place of injectable or oral contraceptives. Condom use for HIV and STI prevention was strongly encouraged.
Written informed consent was obtained prior to data and sample collection. Ring initiation schedules varied depending on completion of last OCP or DMPA-use cycle. OCP users were able to initiate ring use as soon as 30 days following study enrollment, while DMPA users may have had to delay ring initiation up to three months. To avoid overlap use in contraceptives, discontinuation of DMPA and initiation of NuvaRing® were recommended on the due date for the next injection. The informed consent process and data collection were available in the language choice of the participant (English, Swahili, or Dholuo). After receiving instruction and demonstration on a 3-dimensional female pelvic model, ring self-insertion and removal practice training occurred at the study clinic office for 210 women. At each follow-up visit, women received a bar of soap and 500 Kenya Shillings (approximately US$ 5). In addition, as a part of the study clinic’s standard services, women received feminine sanitary pads, condoms, and hormonal contraceptives (at study exit or CVR discontinuation). Participants and their children were eligible to present to the study clinic at any time for diagnosis of common ailments and, if appropriate, referral for treatment. Participants’ sexual partners were entitled to receive free STI treatment following syndromic management assessment.
Data collection
Non-adherence assessment was performed at follow-up visits scheduled between day 21 and day 29 of each one-month CVR cycle for six months following CVR initiation. At each visit, an electronic pharmacy log was used to record dates of each CVR dispensation and return of used CVRs by each participant. Of note, CVR dispensation and follow-up CVR visits occurred on the same date.
Demographic as well as baseline and quarterly behavioral data were collected using audio computer-assisted self-interview (ACASI). CVR user experiences and non-adherence were assessed using an adapted version of the NuvaRing® questionnaire developed by Novak et al,15 administered via computer-assisted personal interview (CAPI). Our questionnaire covered five broad CVR dimensions: difficulty of use, ambiguity of instructions, sexual discomfort, non-compliance, dissatisfaction. Willingness to recommend the CVR to others was also assessed. Within the dissatisfaction dimension, questions centered on specific CVR aspects (e.g., insertion, removal, placement, package use, physical comfort, partner support).
Testing for pregnancy was undertaken at each monthly visit. Pregnant women discontinued CVR use, received local antenatal care clinic referrals, and participated in quarterly follow-up. Rapid HIV testing was performed at baseline and every three months thereafter. Testing for other STIs and bacterial vaginosis was completed at baseline and study exit. Vaginal swabs were used to collect samples for gonorrhea, chlamydia, and bacterial vaginosis testing. Blood samples were collected for herpes simplex virus type 2 (HSV-2) and syphilis testing.
Measures
Our primary outcomes were specified as binary variables summarizing different types of CVR non-adherence based on: (a) self-report, (b) pharmacy record, and (c) residual hormone levels. Participants were classified as non-adherent by self-report if they reported during follow-up any missed CVR use. Non-adherence by pharmacy record was determined if a new ring was not dispensed between days 19-31 for any CVR cycle or a used ring was not returned for every CVR cycle completed.
An objective measure of non-adherence was assessed by analyzing residual etonogestrel (progestin) and ethinyl estradiol (estrogen) levels in returned CVRs for months 1 (n=26), 3 (n=43), and 6 (n=43) for a subset of participants purposively selected from those who self-reported perfect (100%) adherence at all monthly visits (henceforward referred to as the hormone analysis sub-sample). NuvaRing® contains 11.7 milligrams (mg) of progestin, and 2.7 mg of estrogen. The average release rates per 24 hours over the 3-week use cycle for progestin and estrogen are 0.120 mg and 0.015 mg, respectively.16 Participants were classified as non-adherent if returned CVRs showed residual progestin or estrogen levels greater than 95% of those measured in a new, never used ring. Residual hormone levels were defined as consistent if the same use indicators were present across all returned CVRs examined (i.e., 100% overall use or 100% overall non-use).
Responses to demographic questions were coded as categorical variables. Incident STI or bacterial vaginosis, and responses to most risk behavior questions were included as binary variables. CVR dissatisfaction was characterized by a binary variable indicating displeasure with more than three CVR aspects within the four negative attitude domains, which included inconvenience of ring use (including package use), ambiguity of instructions, sexual discomfort, and difficulty with compliance. The number of dissatisfaction aspects reported at each CVR follow-up visit was used to visually depict dissatisfaction over time. Lastly, willingness to recommend the CVR to others was based a 5-point Likert agreement scale (highly agree, agree, undecided, disagree, and highly disagree).
Tolerability was summarized as a binary variable that distinguished between reports of ≥ 2 side effects (SEs) during the CVR-use period and reports of 1 or no SE. SEs included elevated systolic (>160 mmHg) or diastolic (>110 mmHg) blood pressure, self-reported fatigue, vaginal discharge, genital pain, headaches, depression, and abnormal vaginal bleeding. The number of SEs reported at each follow-up visit was also used to graphically depict tolerability over time.
Statistical methods
We examined the factors associated with non-adherence among women who initiated ring use and completed at least one follow-up visit (non-adherence sample), in the sub-sample of participants who provided responses on ACASI questionnaire (behavioral sub-sample), and in the sub-sample of participants included in the residual hormone-level analysis. Descriptive statistics were used to summarize categorical (frequency and percentage) and continuous (mean, median, standard deviation, and range) variables. The association with non-adherence outcomes was summarized by prevalence ratio (PR) and robust 95% confidence interval (CI) estimated from a log-binomial regression model using the generalized estimating equations (GEE) approach.17,18 The same GEE log-binomial regression with the visit number treated as a continuous covariate was used to model the over-time trend in a binary characteristic (dissatisfaction and tolerability). The incidence rate for pregnancy or HIV infection was estimated as the number of respective events occurred over study follow-up per 100 person-years with a robust 95% CI=obtained from a GEE Poisson model. Agreement among non-adherence measures was assessed by Cohen’s kappa.19 All statistical tests were two-sided and interpreted at 0.05 level of significance. The analyses were performed in SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
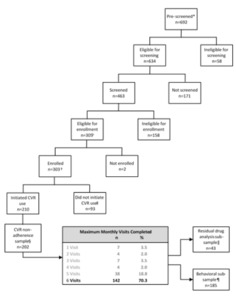
From the women pre-screened, 29.2% (202/692) initiated CVR use and completed at least one follow-up visit (Figure 1). Overall, three HIV seroconversions occurred during the study for an incidence rate of 3.6 per 100 person-years (95% CI=1.2-11.0) (data not shown). Among the non-adherence sample, 91.6% (185/202) who provided responses to ACASI questions were included in the behavioral sub-sample.
The non-adherence sample (n=202) accrued 83.7 out of the expected total 101 (82.9%) person-years of observation. Participants were followed for a median of 5.3 months, with follow-up ranging from 0.7 to 6.5 months (mean=5.0 months, standard deviation=1.1 months). Demographic characteristics of our CVR non-adherence sample are presented in Table 1. Slightly over three-fourths of participants (79.7%) were using DMPA prior to initiating ring use.
Out of the 202 participants, 85.1% (172) self-reported on CVR non-adherence. Overall, 14.0% (24/172) and 54.5% (110/202) were non-adherent by self-report and by pharmacy record, respectively (Table 1). Over a total of 83.7 person-years of follow-up, five pregnancies occurred yielding a pregnancy rate of 6.0 per 100 person-years (95% CI=2.5-14.3) (data not shown).
The only significant factor associated with non-adherence by pharmacy record was the main source of personal income (Table 1). Specifically, the prevalence of non-adherence by pharmacy record was reduced by 29% among women with salary-based income compared to those with no personal income or no income (PR=0.71, 95% CI=0.55-0.91, P=0.008]. CVR dissatisfaction was the only significant factor associated with non-adherence by self-report. Women indicating dissatisfaction with >3 CVR-related aspects (e.g., ring properties, use features) were more likely to be non-adherent by self-report compared to women dissatisfied with ≤3 aspects (PR=2.69 CI=1.31-5.52, P=0.007).
Figure 2 depicts the proportion of participants (n=202) reporting any CVR dissatisfaction by study follow-up visit. Out of 491 dissatisfaction aspects reported across all follow-up visits, sexual discomfort accounted for 33.6% of dissatisfactions (some participants may have reported this aspect at multiple visits).
SEs were reported by 9-14% of participants each month (Figure 3). The three most commonly reported SEs were headache, fatigue, and vaginal discharge. We found no significant associations between CVR tolerability and non-adherence either by self-report or by pharmacy record. All reported SEs were mild in severity (Grade 1) and did not require treatment.
Table 2 displays non-adherence by self-report and by pharmacy record in the behavioral sub-sample (n=185). Out of the 185 participants, 84.3% (156) self-reported on CVR non-adherence. Relevant ACASI data were available for 19 of 24 participants who were non-adherent by self-report and 96.4% (106/110) of participants who were non-adherent by pharmacy record. We found no evidence of an association between the behavioral measures and non-adherence by self-report or by pharmacy record (Table 2).
In the analysis of residual progestin and estrogen, a total of 112 returned CVRs were examined for 43 participants who self-reported perfect adherence at all monthly visits. Overall, 20 (46.5%) out of 43 participants were non-adherent by residual hormone levels, including 7 (16.3%) who showed consistent non-use and 13 (30.2%) who showed partial or inconclusive use (consistent with tampering). Analysis of residual hormone levels in returned CVRs showed increasing non-adherence over time (P=0.004, non-adherence for months 1, 3, and 6 were 19.2%, 32.6%, and 44.2%, respectively).
Finally, we observed poor to no agreement (Cohen’s kappa = 0.03) between pharmacy record and self-reported non-adherence measures. There was disagreement observed between pharmacy record non-adherence and residual drug analysis as well as between self-reported non-adherence and residual drug analysis (data not shown).
DISCUSSION
We sought to understand the drivers of non-adherence to a CVR in a naïve population, with an intent to incorporate lessons learned into future studies of multipurpose HIV prevention rings in western Kenya. Despite all first-time users of CVR in our study being willing to recommend the ring to other women, which suggests that this technology for at least contraceptive purposes may be appropriate in this setting, following the guidelines for CVR use may have been a challenge for some, in particular those lacking a salary-based income or those being dissatisfied with >3 aspects of CVR use. Our sub-analysis of residual hormone levels in returned CVRs suggested increasing ring non-adherence over time. Among our behavioral sub-sample, no statistically significant factors for non-adherence either by self-report or by pharmacy record were found.
Clinical trials, observational studies, and medical practice have shown consistently high levels of satisfaction with NuvaRing®.20,21 In general, women have indicated the ring is easy to use, effective, and convenient, with interference with sexual intercourse and local SEs (e.g., leukorrhea, vaginal discomfort, vaginitis) as the primary reasons cited for either discontinuing or disliking the ring.21,22 Common reasons for disliking the CVR were similar for women in our trial. Presumably, with increased consumer education, as well as an improved understanding of user preferences, these barriers can be overcome.
Non-adherence ranging from 9% to 20% by self-report have been found in other NuvaRing® studies21,23 and was slightly lower (8%) in a recent dapivirine ring HIV prevention trial conducted in multiple African countries.24 Our by-pharmacy-record measure may have under-estimated non-adherence. Other studies have defined NuvaRing® cycle non-adherence as a ring cycle that (a) extended 48 hours beyond day 22, and (b) the length of ring-free period was lengthened by more than 24 hours from day 8.25 Notably, these studies collected diaries and tracking logs completed by the participant as opposed to our method, which involved calculations based on pharmacy dates for dispensation of new CVRs and return dates of a used CVRs. As suggested by others, non-adherence for longer-acting ARV rings require cumulative measurement over time that could benefit from non-invasive electronic and biometric monitoring technologies requiring minimal participant effort.26
Other studies have found decreased non-adherence among women with an independent income.4 Women with their own source of income may have greater family planning decision-making input than women who depend on their partners, or women who have variable income (eg, seasonal, casual, or temporary work). While additional research is needed, economic empowerment interventions suggest that most programs show reproductive health improvements.27
A recent systematic review of contraceptive use in sub-Saharan Africa suggests that misinformation and concerns about perceived SEs are common barriers in accepting and using modern contraceptive methods.28 As measured in our study, we did not find that SE concerns and tolerability were barriers to CVR use.
A number of limitations are associated with this study. The generalizability of our findings is limited due to our study design and sampling approach. While women in our sample were able to use a novel intravaginal ring to prevent pregnancy, which may have implications for future use of rings to prevent HIV and other STIs in this high-burden region, caution is warranted with such an interpretation.
A gold standard for assessing adherence to modern contraceptive methods is not available.29 Our definitions of particular measures, in particular non-adherence by self-report, non-adherence by pharmacy record, may have produced imprecisions with either observation or measurement process. Measures for assessing CVR non-adherence in the literature are largely limited to self-report and subject to under-reporting of non-adherent behavior. We acknowledge that some women in our study could have had on-time CVR dispensation (between days 19 and 31 for each monthly cycle) and returned a used ring; yet, they could have been partially or completely non-adherent. Similar to self-report, a by-pharmacy-record measure is suboptimal in reliably assessing non-adherence. HIV risk perceptions along with HIV stigma, which were not measured in our study, may have created reticence to initiate ring use and influenced non-adherence behavior. Self-reported non-adherence and behavioral data may be subject to recall or social desirability biases. Performing residual hormone analysis on the returned rings from all women in our sample was not feasible. Moreover, such objective measures may not be entirely clear-cut in their ability to distinguish inconsistent users from consistent users.29 Given small count, our pregnancy rate was estimated with low precision and reliability, and should be interpreted with caution. Lastly, NuvaRing® may offer a slight allowance for imperfect use given that removal of the ring for up to three hours for sexual or hygiene purposes does not compromise contraceptive efficacy.
CONCLUSIONS
While vaginal rings may be a highly effective but underutilized contraceptive approach and potentially a preferred future HIV prevention method for some women, perfect use will be a challenge. Apart from measurement challenges of non-adherence in clinical trial settings, real world non-adherence must be carefully accounted for in development of any multipurpose vaginal ring product. Economic empowerment interventions, in particular those that emphasize consistent and partner-independent income options, may mitigate non-adherence to modern contraceptive methods such as a CVR. To better understand non-adherent behavior, it may be necessary to go beyond looking for demographic and behavioral factors to explain CVR non-adherence, such as analyzing participants’ relationship with power dynamics, self-assertion, and sexuality. Lastly, to further minimize non-adherence, preemptively addressing CVR dissatisfaction and expanding education on CVR features may be beneficial.
Acknowledgements
The authors would like to thank the study participants for their time and contribution to this study as well as the broader Kisumu community for its support. The authors would also like to thank Beatrice Nyagol, Kenneth Ondeng’e, Lawrence Opado, Richard Ndivo, and members of the study implementation team. The authors extend a special thanks to Siobhan O’Connor, Clyde Hart, and David Schnabel for their technical guidance and support.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the United States Centers for Disease Control and Prevention (CDC) or the Department of Health and Human Services. The Kenya Medical Research Institute (KEMRI) Director and the KEMRI Publication Review Committee gave permission to publish this manuscript.
Funding
The Centers for Disease Control and Prevention supported this work under PN1757, 2014-2015.
Clinical trial information
The study is registered at ClinicalTrials.gov under the identifier NCT02529683.
Competing interests
The authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no conflict of interest.
Correspondence to:
Eleanor McLellan-Lemal
Centers for Disease Control and Prevention
1600 Clifton Rd., MS-E45
Atlanta, GA 30329
United States of America
[email protected]

_reporting_1_dissatisfaction_aspect_by_kisumu_contracep.png)
_reporting_1_side_effect_(se)_by_kisumu_contraceptive_v.png)

_reporting_1_dissatisfaction_aspect_by_kisumu_contracep.png)
_reporting_1_side_effect_(se)_by_kisumu_contraceptive_v.png)