In comparisons made after pooling data from millions of participants, population mean systolic blood pressure (SBP) in Africa exceeds that of most other regions in the world.1,2 Few population-based studies3 have examined variation in the rates of high blood pressure and associated risk factors within Africa, a massive geographical region with heterogeneous ancestry and culture.
While there are many genetic and environmental determinates of blood pressure, one genetic factor with a large effect on vascular phenotypes is the Apolipoprotein L1 (APOL1) gene. Scientists have linked the disproportionate risk for chronic kidney disease (CKD) among African-Americans to two coding variants (denoted G1 or G2) in the APOL1 gene.4,5 The high frequency of G1 and G2 in sub-Saharan Africa appears due at least in part to protection they provide against the trypanosomes that cause African sleeping sickness. Persons with at least one copy of the G1 or G2 allele (heterozygotes) are protected against *Trypanosoma brucei rhodesiense, the parasite responsible for acute African sleeping sickness.4,6,7 Harboring two risk alleles (homozygosity) is associated with a large increase in the risk of hypertension-attributed kidney disease or focal segmental glomerulosclerosis in African Americans4,5 and in the risk of chronic kidney disease in general in Africans.8,9
Since high blood pressure manifests as an early sign of kidney disease,10 it is conceivable that high blood pressure may be more common in populations with high rates of homozygosity for the APOL1 G1/G2 polymorphisms.11,12 There is wide variation in prevalence of APOL1 G1/G2 variants in ethnic groups across Africa, but the link between blood pressure and APOL1 G1/G2 homozygosity has not been explored in detail in the continent.13 Exploring this link can illuminate the full implications of these gene variants on the health of Africans and inform strategies for kidney disease screening in resource-poor settings. We therefore sought to test the hypothesis that higher prevalence of APOL1 G1/G2 homozygosity among African ethnic groups is associated with higher blood pressure.
METHODS
We identified three data sources for recent population-based studies in Africa with available information on lifestyle risk factors, measured blood pressure, and ethnic group status: the Ghana Demographic Health Survey (Ghana DHS),14 the Kenya World Health Organization stepwise approach to chronic disease risk factor surveillance survey (Kenya STEPS),15 and the cardiometabolic risk survey of rural South Western Yoruba (Nigeria SW).16 These three studies met three criteria: (1) population-based sampling, (2) individual level measured blood pressure, and (3) individual level report of ethnic group.
Using individual level data from these three studies, we were able to extract and harmonize the following: age, sex, education, ethnic group, rural or urban residence, and smoking. Self-reported hypertension status and medication use were only available in Ghana DHS and Kenya STEPS. All three studies measured BP and reported a measured Quételet’s (body mass) index (BMI, kg/m2). Table S1 in Online Supplementary Document shows the definitions of key variables.
We linked ethnic group as reported by participants in each study to the ethnic group’s APOL1 genotype frequency as described by Thomson et al. (6). Our analytic group is therefore comprised of persons from each study (total n=20 284) with available data on ethnic groups’ APOL1 G1/G2 allele frequency (analytic group n=10 423).
To approximate prevalence of G1/G2 homozygosity, we assumed Hardy Weinberg Equilibrium; calculations assuming Hardy Weinberg Equilibrium closely matched genotyped frequency in three ethnic groups (Table S1 in Online Supplementary Document). Compound heterozygotes (with one G1 and one G2 allele) were treated the same as G1/G1 and G2/G2 homozygotes and all three genotypes are considered “high risk genotypes,” as many studies have demonstrated.4,17,18 We were able to create eight discrete data points for APOL1 G1/G2 homozygosity frequency.
Statistical analysis
We describe means and proportions of harmonized characteristics by study country. For comparison across studies, we report mean SBP and hypertension prevalence after age-standardization to the World Health Organization World Standard population (2000-2025).19
We performed linear regression with mean SBP as the outcome, and linear G1/G2 homozygosity as the principal exposure of interest, adjusting for available individual level risk factors and country of survey. We performed the same analyses for DBP as the outcome. In the Ghana and Kenya subsets, we tested for an interaction between medication use for hypertension and G1/G2 homozygosity; since the p value for interaction term exceeded 0.05, we dropped this term from the final reported model. Finally, we report adjusted mean (95% confidence interval (95% CI)) SBP by the G1/G2 homozygosity of ethnic groups with available data, computed as averages among the actual population using the margins command in Stata.20
Missing data
Overall, 2553 (24.5%) out of 10 423 records were incomplete. The outcome variable (systolic blood pressure) was missing for 68 (0.6%) patients but most missing data were due to BMI (missing for 35% of the patients from Ghana). After an extensive review of the Ghana DHS methodology we concluded that the data were missing at random, ie, values were not missing because of a reason related to the missing data itself. Table S2 in the online supplementary document reports participant characteristics by missing versus non-missing BMI status. We used multiple imputation with a joint normal approach21,22 to impute 35 data sets. The imputation model included all covariates in the analysis model, BMI and the outcome.23 Model parameters were estimated applying the analysis model to each imputed data set separately. These estimates and their standard errors were combined using Rubin’s rules.
We used SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) and Stata version 13.1 (StataCorp. 2013. College Station, TX, USA) to perform all analyses.
RESULTS
After harmonization of data from the three population-based studies, Table 1 describes the characteristics of participants with available data on ethnic group APOL1 G1/G2 genotype frequency. The Kenya (n=2591) and Ghana (n=6443) studies had similar age distributions and proportion of male participants; participants from Nigeria (n=1389) were more likely to be older and male. Kenyan participants reported higher smoking and illiteracy rates. Mean SBP and hypertension prevalence was highest in Kenya. The differences attenuated after age-standardization with mean SBP 130.2 (standard error (SE) 0.4) mmHg, 121.9 (SE 0.5 mmHg), 120.3 (SE 0.8 mmHg) and hypertension prevalence 36.0%, 24.7% and 20.6% in Kenya, Ghana, and Nigeria respectively.
Age, sex, BMI, and homozygosity rates for the APOL1 G1/G2 alleles were associated with mean SBP (Table 2). For each 10 year increment of age, mean SBP was higher by 4.4 mmHg (95% CI 4.1 to 4.7 mmHg). Women had 6.8 mmHg (95% CI -7.5 to -6.0 mmHg) lower mean SBP. Country of survey was also strongly associated with mean SBP; persons in Kenya STEPS had 19.8 mmHg (95% CI 16.9 to 22.7 mmHg) higher adjusted mean SBP and persons in Ghana DHS had 5.9 mmHg (95% CI 4.2 to 7.6 mmHg) higher adjusted mean SBP compared with persons in the Nigeria SW study.
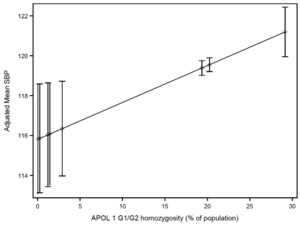
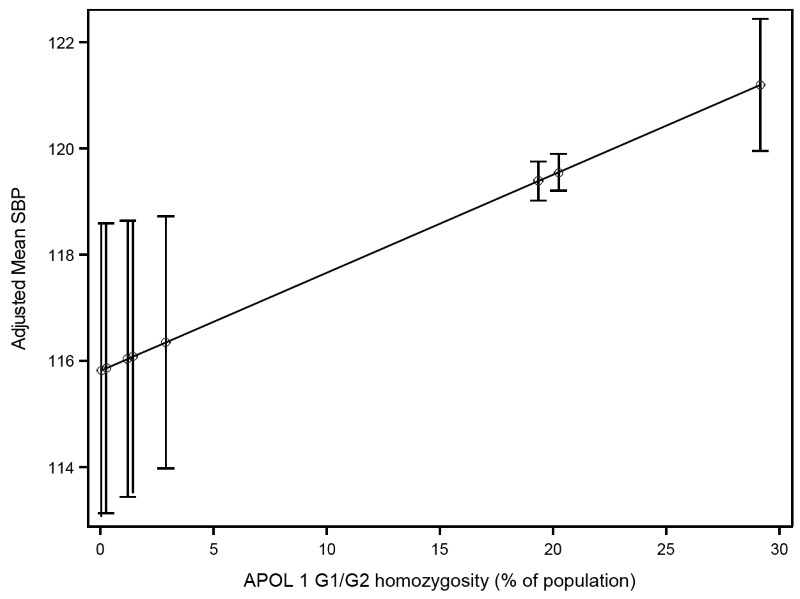
Adjusted mean SBP according to APOL1 G1/G2 homozygosity for each ethnic group are presented in Figure 1. Table S3 in Online Supplementary Document describes ethnic groups associated with each G1/G2 recessive trait, frequency of persons, prevalence of G1/G2 homozygosity in each ethnic group. Mean SBP in the group with the highest G1/G2 homozygosity (29.2% in Akan, Ghana) was 5.4 mmHg higher than in the group with the lowest frequency (0.04% in Somali and Borana in Kenya). There was a trend toward higher mean DBP with higher APOL1 G1/G2 homozygosity (Table S4 in Online Supplementary Document).
DISCUSSION
In our cross-country study evaluating the association of an ethnic group’s frequency of APOL1 G1/G2 homozygosity with blood pressure, we found that after adjustment for common risk factors, mean SBP was 5 mmHg higher in ethnic groups where nearly a third of persons carry the high risk APOL1 alleles (in our study, the Akan people in Ghana), compared with ethnic groups where homozygosity is rare. In our model, the estimated effect of APOL1 G1/G2 homozygosity on mean SBP was on par with the estimated effect of 12 additional years of age.
This finding confirms on a population level published individual level data linking APOL1 and blood pressure. Data from the Dallas Heart Study, a population-based study in the US with oversampling of African Americans, showed a higher prevalence of hypertension and 2.8 mmHg higher mean SBP in persons carrying two APOL1 risk alleles.18 A recent analysis24 by Nadkarni et al. collating individual level data from multiple centers within the US also showed approximately 2 mmHg higher SBP among persons with two copies of the APOL1 risk alleles, compared with persons with no copies and after adjustment for age, sex, and estimated glomerular filtration rate. The estimated effect on DBP was smaller, as was seen in our study.
Interestingly the Nadkarni et al. study also noted a 1mm Hg higher SBP with even one copy of APOL1 G1/G2 allele, although higher kidney disease risk has been observed almost exclusively to follow a recessive model. In addition, the elevated blood pressure attributable to APOL1 genotype occurred long before changes in serum creatinine, which is the traditional, although imperfect, measure of kidney disease. These authors24 and others25 have proposed that expression of risk variant APOL1 protein in the extra-renal vasculature could predispose to higher blood pressure and higher risk for cardiovascular disease, independent of underlying kidney function. In contrast, a recent analysis of blood pressure trajectories after nearly 25 years of follow up, Chen et al.26 found that blood pressure was similar across APOL1 G1/G2 high versus low risk gene variants in African Americans; two recent analyses did not find any differences in incident intermediate cardiovascular disease measures27 or prevalent cardiovascular disease.28 Analyses of whether APOL1 gene variance affects cardiovascular phenotypes such as cardiac disease and stroke have been inconsistent and may relate to the study of cohorts designed to answer other, unrelated questions or to differences in inclusion and exclusion criteria.25,29-30
Whether APOL1 G1/G2 homozygosity or heterozygosity leads to higher blood pressure without underlying kidney disease remains in debate, but once a person develops early kidney disease (eg, albuminuria), he/she is at substantially higher risk for development of elevated blood pressure.31-32 Regardless of the mechanism by which APOL1 G1/G2 variants are associated with high blood pressure, we believe our findings and others’13 have two implications for health policy makers in Africa. First, management of blood pressure can be key element in health care planning when considering the overall health effects of APOL1 nephropathy, especially in light of evidence suggesting survival benefit with strict blood pressure control among African Americans with high blood pressure and APOL1 G1/G2 homozygosity.33 Second, high blood pressure could serve as an initial warning sign to investigate underlying kidney disease among persons who belong to ethnic groups with high rates of APOL1 G1/G2 homozygosity. Among those persons, there may be a higher-than-usual probability that high blood pressure is mediated via impaired kidney function.
Our analysis has several strengths. We obtained data on measured blood pressure in large population-based studies, with data on a range of ethnic groups with large variation in APOL1 G1/G2 homozygosity rates. We were able to adjust for several important individual level determinants of blood pressure, including age and BMI. While we adjusted for urban versus rural residence, which can serve as proxy for lifestyle factors influencing blood pressure, we could not harmonize data on physical activity and diet. We found higher mean adjusted SBP in Kenya compared with Ghana and Nigeria which could be due either to residual confounding from differences in lifestyle factors or income among participants, or to the different methodologies of BP measurement in each survey. In another population based study performed in a Nairobi slum, the age-standardized prevalence of hypertension was 23%,34 closer to the prevalence for hypertension we found in Ghana and Nigeria, and lower than in subset of the Kenya STEPS study with available data on ethnic group (who generally have lower G1/G2 APOL 1 homozygosity) used in our analysis. Other potential genetic explanations for higher blood pressure in African ancestry populations have also emerged, but were not explored here.35
In summary, our evaluation of the association between APOL-1 polymorphism and blood pressures shows that ethnic groups with higher frequency of the G1/G2 traits have higher adjusted mean SBP. This analysis implies that blood pressure and the genotypic frequency of APOL-1 polymorphism in populations could be used to guide kidney disease surveillance strategies in Africa. Catastrophic vascular effects related to uncontrolled blood pressure can occur prior to clinically-evident kidney disease, and we suggest that policy makers in Africa expand their view to include the many potential health effects of APOL 1 G1/G2 variants in their populations.
Acknowledgements
Dr Shuchi Anand is funded by NIDDK K23 5K23DK101826-04. We thank Dr Gretchen Stevens at World Health Organization for her assistance with identifying collaborators.
Disclaimer
The data analysis performed herein was done solely by the authors, using the publically available Demographic Health Surveys or de-identified data from Kenya STEPS and Nigeria rural Yoruba study.
Funding
US National Institutes of Health, US Agency for International Development, Kenyan Ministry of Health.
Competing interest
The authors completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available upon request from the corresponding author), and declare no conflict of interest.
Correspondence to:
Dr Shuchi Anand Stanford University/Nephrology 777 Welch Rd, Ste. DE Palo Alto, CA 94034 USA [email protected]