Chronic Respiratory Diseases (CRD), most commonly asthma and chronic obstructive pulmonary disease (COPD), but also post -tuberculosis (TB) lung disease, bronchiectasis, interstitial lung disease (ILD), including post-covid sequel are common public health challenges, with high prevalence and mortality rates globally, especially in low- and middle-income countries (LMICs).1–3 Symptoms such as cough, phlegm, shortness of breath, chest tightness and wheezing are disabling features of CRDs that contribute to poor health-related quality of life, negative impact on family, work and societal roles, and stretching utilisation of healthcare resources. CRDs contribute to a very high economic burden.4
Despite the high morbidity and mortality of CRDs, awareness of the true economic and societal impact of CRDs in LMICs is often low, with limited robust data on the true burden of disease in these countries. Underdiagnosis and incorrect diagnosis are the major hurdles in getting the true prevalence of these conditions, which further makes it complicated by debates over using spirometry thresholds (fixed ratio vs lower limit of normal) and predictive value of (largely non-specific) respiratory symptoms.5 Further, those diagnosed or diagnosed lately are not managed and treated appropriately due to low awareness and knowledge among the general practitioners.6,7
Additionally, there are a few more issues which make it difficult to combat CRDs, such as a) Non-availability of spirometry (considered the gold standard in CRD diagnosis) for screening and diagnosis of CRDs, especially at rural, semi-urban, remote, tribal level; b) High cost of PFT services in private health sector making it unaffordable for rural & urban poor (a full PFT costs around USD 14.40); c). Long distances hampering accessibility - multiple visits, loss of wages, poor compliance, cost of travel, lodging, etc. District hospitals have a long waiting time due to the large number of patients and inadequate manpower and infrastructure to cope. This leads to poor health outcomes due to a lack of appropriate and timely interventions at the primary care level in India.8,9
While spirometry is considered a Global Initiative for Chronic Obstructive Lung Disease (GOLD) standard for COPD and Asthma diagnosis, there is a caveat on the application of spirometry. The information obtained on spirometry may be misleading if meticulous quality control is not exercised in equipment selection and maintenance, calibration, operator training and competence, and patient performance.10 It is also imperative that prediction equations developed in the local population are used to interpret data and avoid misclassification that is inevitable if inappropriate equations are used. The equations for the Indian population, using the current standardisation of spirometry, have been published recently.11
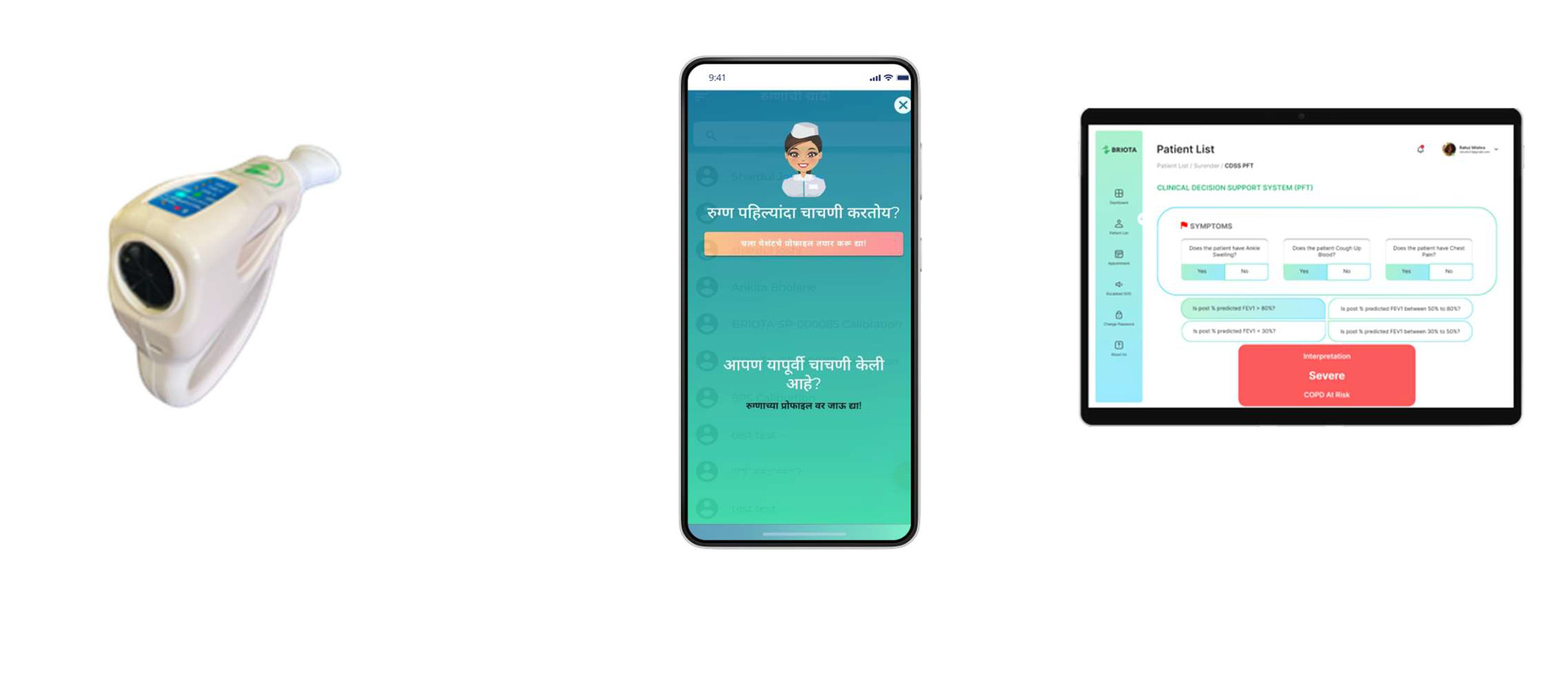
This gap in primary care has led to the advent of innovative digital “Make in India” solutions like Briota PFT in a Box™. This unique point of care solution includes a handheld digital spirometer SpiroPRO®, AI (Artificial Intelligence) first clinical decision support system software CDSS and remote spirometry verification. This point of care solution has been designed and validated to meet the unique needs of CRD management in primary healthcare settings. It is portable, cost-effective, and capable of providing accurate and immediate results while reducing the need for frequent device calibration, and specialised technical expertise.
Briota PFT in a Box™ is used in the SAVE™ (Spirometry Assisted Virtually Early™) program. SAVE™ is first of its kind holistic program for CRD screening, diagnosis and management at primary healthcare for rural India. SAVE™ is designed as a community health program based on consultations with various stakeholders in community and primary health care. These stakeholders included representatives of the non-communicable disease cell, Ministry of Health and Family Welfare Government of India, various state health departments in India and K.E.M. Health Research Center Pune, Maharashtra, India. A cross sectional study covering 15,602 participants from 42 villages in Dindori subdivision of Nashik district, Maharashtra state was conducted as part of program SAVE™.
METHODS
This study was performed as part of program SAVE™. Program SAVE™ has a 5-step strategy for CRD management: 1. assessment – house to house survey 2. screening – 4 parameter expiratory only spirometry test 3. Diagnosis – 15 parameter pre and post bronchodilator spirometry test 4. CDSS assisted CRD diagnosis and treatment at primary healthcare 5. Expert consultation. In this study, the same 5 steps were following. First our team of locally hired and expertly trained SAVE™ coordinators successfully executed a house to house survey using a mobile app. This survey covered community-based assessment checklist (C-BAC) questionnaire, encompassing 2 sections – Group A (generic risks for non-communicable diseases) and Group B (specific risks for CRDs and symptoms). The survey was conducted house to house. In many villages the survey was completed with the help of community health workers - Accredited Social Health Activist (ASHA). Prior to the scheduled day of survey, a team of SAVE™ coordinators visited the village and shared details on program SAVE™ with the local administrative body, displayed posters, distributed pamphlets, and conducted community meetings with the villagers with support from ASHA. On the day of the survey, SAVE™ coordinators-initiated house to house surveys in each household starting from a central part of the village. Verbal consent was obtained from the head of the family or the senior most member present in the house before asking any questions to the family. In cases where there were refusals, the residence was locked, or no adult members were available at the time of the survey, the next house was approached. SAVE™ coordinator first administered C-BAC survey Group A, which included questions related to generic risk factors for non-communicable diseases (NCDs) and information about any existing chronic diseases. Based on the responses, a risk assessment score “Group A” was generated by the mobile application. Participants with a high-risk score were further administered with questionnaire Group B, specifically designed for screening of CRDs. Group B included information about smoking habit, CRD symptoms, breathlessness, mucus production, wheezing, reduced physical activity, fitness for spirometry etc. Based on the responses, a new risk score “Group-B” was generated. Participants were provided with printouts of healthy lifestyle and, smoking and tobacco cessation guidelines. Based on the risk score “Group-B”, participants were administered a 4-parameter expiratory only spirometry test at the house or at nearest community health center. This test was conducted using Briota SpiroPRO® device and mobile application. Each participant who has taken the 4-parameter spirometry test, was provided with a SAVE™ CRD screening card. This card displayed a Red, Yellow or Green classification color code. Appropriate classification color code was marked based on the report generated in the mobile application . If a particular participant was marked with Red or Yellow color classification code, he/she was scheduled for a pulmonary function test (PFT) examination at the PHC. If the participant was provided with a CRD screening card with Green color classification code patient was advised on healthy lifestyle and smoking cessation if applicable.
When participant visited primary health care center with a Red or Yellow colour classification CRD screening card for PFT test, SAVE™ coordinator, trained in Spirometry administered a 15-parameter pre and post bronchodilator spirometry test. Spriometry was administered using a calibrated spirometer device SpiroPRO®, as shown in figure 1 (panel a). 15 minutes after administration of Salbutamol 400µg, reversibility was tested for pre and post bronchodilator conditions. The PFT report was generated on the mobile application and was available in Briota CDSS system. SAVE™ coordinator or a nurse entered clinical history for each patient in CDSS. Medical officer performed clinical examination and answered questions in Briota CDSS. Briota CDSS then provided probable CRD diagnosis and recommended treatment path for each patient. This CRD diagnosis and treatment path was cross checked by the chest physician available at district hospital in person or via a tele-conference. Briota CDSS provided lung health score (LHS™) for each patient. LHS™ was generated based on the proprietary machine learning models for clinical evaluation, spirometry tests, clinical investigations, medical examination findings, disease classification etc. The LHS™ was further used as a reference point during disease management phase. Briota CDSS adheres to guidelines of Global initiative for chronic Obstructive Lung Disease (GOLD) for COPD and Global Strategy for Asthma Management and Prevention (GINA) for Asthma.
Each confirmed diagnosed patient was offered a patient support program – Systematic Intervention Agent (SiA®) program included training sessions on inhaler technique and breathing exercises.
SAVE™ coordinators and medical officers were trained by a local team of researchers and chest physicians to conduct PFT according to the ATS and European Respiratory Society (ERS) guidelines12 and on how to use Briota SpiroPRO® and Briota CDSS.
Various forms were filled and records maintained by SAVE™ coordinators at various stages of participant flow. This data was compiled on a weekly basis and a regular review was conducted for maintaining data sanctity.
Participants were excluded from the 4-parameter and PFT spirometry test for safety concerns, if they:
-
were seriously ill
-
were unable to comprehend or had any contraindication to perform spirometry
-
faced severe injury
-
received surgery to abdomen, chest, and/or eye in the past 3 months
-
had myocardial infarction in last 3 months
-
were hospitalised due to any cardiac illness in last 90 days
-
were on treatment for tuberculosis
-
have any symptoms of tuberculosis
-
pregnant
If recommended by a medical officer, participants with active symptoms of tuberculosis (TB) were provided with an X-ray via a portable digital X-ray unit. If TB suspected then the participant was further referred for TB diagnosis as per TB screening and diagnosis protocol of national TB elimination program (NTEP).
Setting
This study was conducted in Dindori Taluka (subdivision) of Nashik District of Maharashtra state in India. The majority (55%) of the population of Dindori Taluka is tribal (15). This study focused on 42 out of 158 villages, in the Dindori Taluka, covered by 10 primary health centres in consultation with the District Health Officer (DHO) of Nashik district and the office of Minister of State, Ministry of Health and Family Welfare, Government of India.
. Subsistence farming and farm-labour are the main occupations in Dindori. There are very few private medical practitioners and the healthcare is predominantly provided through the public healthcare system which includes 1 rural hospital, 10 primary health centres and 78 sub-centers and is integrated with district hospital Nashik, Maharashtra state India (16).
SAVE™ coordinators’ recruitment and training
30 SAVE™ coordinators from Nashik District, Maharashtra state, India were trained and employed under program SAVE™ for conducting the survey. All SAVE™ coordinators received PFT training from PFT experts and chest physicians.
Questionnaire
Informed by the literature review conducted,10,11 a range of validated questionnaires that had previously been used in LMICs to detect CRDs were considered. A participant with a high-risk score for Group A was further administered Group B questionnaire (Supplementary file 2 questionnaire B). We pilot-tested the questionnaires before actual use for the survey. Inputs and insights from various stakeholders, such as chest physicians, public health officials, senior scientists and researchers from community health, were also incorporated for the development of the survey questionnaires.
Data entry
E-data were collected on the android mobile app as shown in figure 1, panel b using the survey questionnaires. Spirometry data was transferred over bluetooth from SpiroPRO® device to mobile app, which further transferred the data to a centralised server shown in figure 1, panel c. For data analysis, spirometry values were exported from the centralized server into excel files. Medical officers, nurses and SAVE™ coordinators made entries for clinical examination, symptoms, medical history etc. in Briota CDSS. Spirometry report printouts with corresponding graphs were available for quality checks and verification of the data.
Sample size calculation
Based on discussions with District Health Officer (DHO) and chest physician of district hospital, with an objective of diagnosing 1000 confirmed CRD patients, considering CRD prevalence of 10% and non-responsiveness and dropout rate of 33% during various stages of diagnosis, a sample size of 15000 participants was selected for conducting a c-bac (community-based assessment checklist) survey for CRD risk stratification. The study was conducted among all adults and children 12 years and above residing in the selected villages.
Patient support program
After the patient’s CRD diagnosis was confirmed, the patient was counselled and trained by SAVE™ coordinators on medicine adherence, diet, exercise, and smoking/tobacco cessation. SAVE™ coordinators were involved in counselling and follow-up of these participants for treatment and management. Patients who agreed to enroll in the Systematic Intervention Agent (SiA®) patient support program were offered regular remote and in-person exercise, diet and mental health sessions. Out of the 1,154 patients with a confirmed diagnosis of CRD, based on various criteria such as willingness and availability of a smartphone at home for a video call, 712 patients (61.6%) were enrolled in Briota’s patient support and remote care program Systematic Intervention Agent - SiA® . Out of the 712 patients offered this program, 537 (75.4%) patients continued with it beyond the initial trial period of one month, whereas 175 patients (24.6%) dropped out after the trial period of 1 month.
Statistical analysis
Statistical analysis was carried out using Stata version 15 software. NCD risk factors and CRD risk factors were grouped together and analyzed for low/medium/high risk for CRD based on questionnaire A and questionnaire B respective. Respiratory symptoms were grouped and analyzed as persons with chronic respiratory symptoms and persons without chronic respiratory symptoms. Sociodemographic profile and clinical profile were reported as mean (standard deviation) or number (percentage). Spirometry parameters FVC, FEV1, FEV1/FVC ratio was entered as a number. Briota CDSS diagnosis was entered as COPD – A, B, C, D, E based on GOLD diagnosis guidelines, Asthma – mild, medium severe and PTLD – mild, medium, severe. Lung health score calculated by Briota CDSS LHS™ was entered as a number. Briota CDSS diagnosis was evaluated and compared with final diagnosis for each confirmed diagnosed patient based on survey data, spirometry reports, clinical examination report, teleconsultation reports and Lung health score (LHS™) for that particular patient.
A multivariable logistic regression model was used to look for association between Briota SpiroPRO® spirometry results, Briota CDSS diagnosis outcome, Final confirmed diagnosis and other sociodemographic and clinical factors.
Ethical considerations
The study was approved by public health authorities of Dindori Taluka, Nashik District, Maharashtra State, India represented by District Health Officer and Chief Executive Officer of Nashik District as part of program SAVE™. The guidelines for CRD screening, diagnosis, management as outlined in NCD Revised Operational Guidelines 2023-30 released by the Ministry of Health and Family Welfare, Government of India were referred by the authorities for the ethical consideration and approval.
The patients have given their verbal consent for their images, videos and other clinical information to be used for the purpose of study . The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity at image and video level, but anonymity cannot be guaranteed.
RESULTS
Participant survey and screening
We surveyed a total of 15,602 participants based on the study questionnaire Group A and Group B. Total 4,937 (31.6%) participants were risk stratified as a high risk for CRD. These 4,937 participants were scheduled for the 4-parameter spirometry tests (expiratory only) at their house or the nearest community health center.
In the initial 90 days 2,987 (60.5%) participants attended the scheduled appointments. 128 (4.3%) participants from the scheduled appointments were reported unfit for the spirometry test. A 4-parameter expiratory spirometry test was successfully administered for 2,859 (95.7%) participants.
Based on the symptoms and 4-parameter spirometry test results, participants were categorized into 3 categories for CRD probability: 1. green—healthy lungs—1012 (35.4%) participants; 2. yellow—medium probability for CRD—743 (26%); 3. red—high probability for CRD—1104 (38.6%). Participants under the red and yellow categories were asked to visit the designated primary health care center (PHC).
All of these 1847 participants (100%) visited the designated primary health care center (PHC) for a PFT test.
Participants who were administered the full 15 parameters pre- and post-bronchodilator PFT test, 1,539 participants (83.3%) could perform PFT test with an acceptable quality of spirometry as per ATS/ERS guidelines. Briota CDSS based on PFT test report and symptoms categorized these participants into CRD suspect and healthy candidate. In total 1231 out of 1539 i.e. 80% participants were marked in one of the CRD categories by Briota CDSS. T these participants were also examined by the medical officer, further examined by a chest physician in a tele- consulted or in person
The demographic characteristics, risk factors, and chronic conditions present in the study participants who were surveyed on the field using questionnaire A is given in Table 3. The demographic characteristics and risk factors present chronic conditions in the study participants who were at high risk and surveyed on the field using questionnaire A is given in Table 4. The high-risk participants were further surveyed for risk factors, presence of chronic conditions and presence of risk factors using the questionnaire B. They were further assessed for PFT eligibility. Table 5 shows the details of the same.
Confirm diagnosis
Out of the 1,539 participants who had acceptable PFT tests, 1,154 (74.98%) had a diagnosis of CRD confirmed by the clinician based on the data from the questionnaire, spirometry report, Briota CDSS assessment, and clinical examination. 268 (17.4%) participants were confirmed healthy by the clinician, and 117 (7.6%) were reported non-conclusive or were recommended for further investigation or a consultation with a specialist. Table 6 gives details of the count at each level. Figure 1 gives an overview of the activity or processes performed at each stage and the number of participants.
In summary, out of 15,602 adults surveyed, total 4,937 (31.6%) were identified as “CRD high risk”. 1231 participants based on medical examination, spirometry tests and software analysis were identified as CRD candidates by medical officers at primary care. 1154 participants out of 15,602 (7.4%) were confirmed diagnosed as CRD patients post independent evaluation by chest physicians. At the time of follow-up, 537 patients (75% of 712 patients enrolled in patient support program) reported improvement in symptoms and high satisfaction with the program. District health officer, Medical officers, nurses, Accredited Social Health Activist (ASHA) from the primary health care centers confirmed ease of use and feasibility of using Briota PFT in a Box™ in program SAVE™. Outcome and learning from program SAVE™ was documented and submitted to Ministry of Health and Family Welfare Government of India.
DISCUSSION
Learnings from the study
Healthcare staff including ASHA, nurses, medical officers and chest physicians at community health centers, primary health care centers, rural hospital and district hospital confirmed that Briota SpiroPRO® and Briota CDSS widely simplified the early screening and diagnosis of CRDs. Chest physicians confirmed the quality and standards of various processes followed for screening and diagnosis of CRD patients. In a short duration of 90 days, a total of 1154 patients were confirmed diagnosed for CRD based on assessment of a large pool of 15,602 participants which confirms the feasibility of conducting spirometry at community and primary health care level. This study also highlights the benefits for the public health system by using a comprehensive and holistic approach, following well defined processes and using an easy to operate technology for CRD management at community and primary health care level.
Summary of the findings
Survey questionnaires picked up a significant number of “CRD risk high” 4,937 (31.6%) participants from the total surveyed sample of 15607 participants. Out of these CRD high-risk participants – 1539 (31%) completed the entire cycle for diagnosis confirmation. Medical officer at primary health care center has marked 1231 participants as CRD candidates based on Briota CDSS analysis. Independent analysis by a chest physician confirmed 1154 (7.4% of total surveyed population) participants as CRD diagnosed confirmed. This finding confirms the prevalence of CRD in Dindori, Nashik, Maharashtra state, India to be more than or equal to 7.4%. This high prevalence and diagnosis confirmation of CRDs also underlines the feasibility for conducting quality spirometry at community and primary health care level.
Strengths and limitations
This is one of the first of its kind large scale study for assessing feasibility of spirometry and related methodologies in CRD screening, diagnosis and management at community and primary health care level in rural and tribal settings in Maharashtra state, India. It’s evident that the program SAVE® and Briota SpiroPRO® could bring a unique opportunity to improve early screening and diagnosis rates for CRDs in community-based settings, especially in countries like India. Along with PFT, we showed that the pre-screening questionnaire adds to picking up the suspected or high-risk cases. Briota’s hardware and software application (PFT In a Box™ with SpiroPRO®, Briota CDSS, Briota SiA®) provides integrated programs/applications for screening, diagnosing and managing patients with CRDs. SAVE™ coordinators were locally trained and recruited from the respective villages, which helped build a good rapport with the community. However, we carried out the study with a medical program-based methodology, rather than an entrenched scientific research-based methodology. This may hinder with types of data analysed and generalizability to other countries or groups of diseases. Nonetheless, this study focuses only on CRDs, so, a program-based model can be safely accepted.
Interpretation and implications for future studies
It is challenging to conduct PFT at the primary health care - PHC level. PFT test requires trained manpower to perform a quality test and requires an experienced clinician to interpret the spirometry results. Briota SpiroPRO® under program SAVE™ provides an easy-to-adopt point of care and comprehensive solution with a complete program execution methodology and implementation approach including training of technicians, training of local field staff at sub center and PHC, training of patients; manoeuvring quality checks on the device (audio beep noise and a 6-second LED indicator); quality checks on mobile application; symptoms and correlation with CDSS developed as per GOLD and GINA guidelines; clinical examination; remote consultation with pulmonologist; local language for application etc. Briota’s approach to designing and developing best practices for democratising PFT in Indian community settings is a right step towards making CRD screening, diagnosis and management possible at community and primary care level.
Briota has also successfully addressed the major challenge related to availability of trained spirometry technicians by adopting a “PFT as a service” approach and by providing specially designed training programs for local settings. Project SAVE™ is an excellent example of practical and effective use of innovative Make In India technology in empowering public health system for addressing infrastructure and workforce challenges.
Briota SpiroPRO® under program SAVE™ provides an easy-to-adopt point of care and comprehensive solution with a complete program execution methodology and implementation approach including training of technicians, training of local field staff at sub centre and PHC, training of patients; manoeuvring quality checks on the device (audio beep noise and a 6-second LED indicator); quality checks on mobile application; symptoms and correlation with CDSS developed as per GOLD and GINA guidelines; clinical examination; remote consultation with pulmonologist; local language for application etc. This approach to designing and developing helpful practicices for democratising PFT in the Indian community levels is a step towards making CRD screening, diagnosis and management possible at community and primary care level.
CONCLUSIONS
As an outcome, screening and diagnosing CRDs , especially COPD, Asthma and PTLD at an early stage is feasible at community and primary healthcare settings. Introducing the SAVE™ program as a standard intervention for CRD screening and diagnosis can help save huge direct and indirect costs for both patient and public health systems. In addition, SAVE™, using innovative and Make-In-India technology, provides an opportunity to every patient, even in rural, tribal and remote areas, to get assessed, screened and diagnosed early for CRDs. Several CRD patients have been and will be helped as an outcome of this study.
Acknowledgements
We gratefully acknowledge various stakeholders for program SAVE™ and this present study in Dindori, Nashik district, Maharashtra state India. These stakeholders include local staff, medical officers, chest physicians of the public healthcare system – sub -center, PHC, district hospital, rural hospital; officials and staff of Nashik zilla parishad; research teams at Indira Gandhi Government Medical College & Hospital (IGGMC), Nagpur, Maharashtra, India; research teams at Symbiosis Medical College for Women (SMCW), Lavale, Pune; research teams at Symbiosis International University (SIU), Pune, Maharashtra, India; community health research teams at KEM HRC Vadu
Financial support and sponsorship
This study for Dindori, Nashik, Maharashtra state India was sponsored by Centre for Innovation and Entrepreneurship (CIE) IIIT Hyderabad, and Briota Technologies Pvt Ltd.
Competing interests
In accordance with JOGHR policy and our ethical obligations, GS and SJ reported to JOGHR that they both have share holding in Briota technologies pvt ltd, a company that may be affected by the research reported in the enclosed paper.
CORRESPONDENCE TO:
Gajanan Sakhare, PhD | Briota Technologies Pvt Ltd | Pune | Maharashtra | India 411041 [email protected]
Authorship contributions
Conceived and designed the study – GS, YC; Collected data – GS, SJ; Analysed data – YC, RM, MB; Wrote the manuscript – GS, YC, RM, RB; Revised the draft critically for intellectual content – GS, SJ.